KCC2 Regulates Dendritic Spine Formation in a Brain-Region Specific and BDNF Dependent Manner
- PMID: 30169756
- PMCID: PMC6188549
- DOI: 10.1093/cercor/bhy198
KCC2 Regulates Dendritic Spine Formation in a Brain-Region Specific and BDNF Dependent Manner
Abstract
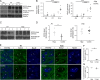
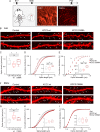
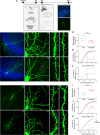
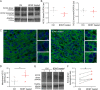
KCC2 is the major chloride extruder in neurons. The spatiotemporal regulation of KCC2 expression orchestrates the developmental shift towards inhibitory GABAergic drive and the formation of glutamatergic synapses. Whether KCC2's role in synapse formation is similar in different brain regions is unknown. First, we found that KCC2 subcellular localization, but not overall KCC2 expression levels, differed between cortex and hippocampus during the first postnatal week. We performed site-specific in utero electroporation of KCC2 cDNA to target either hippocampal CA1 or somatosensory cortical pyramidal neurons. We found that a premature expression of KCC2 significantly decreased spine density in CA1 neurons, while it had the opposite effect in cortical neurons. These effects were cell autonomous, because single-cell biolistic overexpression of KCC2 in hippocampal and cortical organotypic cultures also induced a reduction and an increase of dendritic spine density, respectively. In addition, we found that the effects of its premature expression on spine density were dependent on BDNF levels. Finally, we showed that the effects of KCC2 on dendritic spine were dependent on its chloride transporter function in the hippocampus, contrary to what was observed in cortex. Altogether, these results demonstrate that KCC2 regulation of dendritic spine development, and its underlying mechanisms, are brain-region specific.
Figures

Similar articles
-
Reducing premature KCC2 expression rescues seizure susceptibility and spine morphology in atypical febrile seizures.Neurobiol Dis. 2016 Jul;91:10-20. doi: 10.1016/j.nbd.2016.02.014. Epub 2016 Feb 10. Neurobiol Dis. 2016. PMID: 26875662
-
An ion transport-independent role for the cation-chloride cotransporter KCC2 in dendritic spinogenesis in vivo.Cereb Cortex. 2013 Feb;23(2):378-88. doi: 10.1093/cercor/bhs027. Epub 2012 Feb 17. Cereb Cortex. 2013. PMID: 22345354
-
K-Cl Cotransporter 2-mediated Cl- Extrusion Determines Developmental Stage-dependent Impact of Propofol Anesthesia on Dendritic Spines.Anesthesiology. 2017 May;126(5):855-867. doi: 10.1097/ALN.0000000000001587. Anesthesiology. 2017. PMID: 28301408
-
Stress and trauma: BDNF control of dendritic-spine formation and regression.Prog Neurobiol. 2014 Jan;112:80-99. doi: 10.1016/j.pneurobio.2013.10.005. Epub 2013 Nov 6. Prog Neurobiol. 2014. PMID: 24211850 Review.
-
Role of the BDNF-TrkB pathway in KCC2 regulation and rehabilitation following neuronal injury: A mini review.Neurochem Int. 2019 Sep;128:32-38. doi: 10.1016/j.neuint.2019.04.003. Epub 2019 Apr 12. Neurochem Int. 2019. PMID: 30986502 Review.
Cited by
-
BDNF signaling during the lifetime of dendritic spines.Cell Tissue Res. 2020 Oct;382(1):185-199. doi: 10.1007/s00441-020-03226-5. Epub 2020 Jun 14. Cell Tissue Res. 2020. PMID: 32537724 Free PMC article. Review.
-
Rett and Rett-related disorders: Common mechanisms for shared symptoms?Exp Biol Med (Maywood). 2023 Nov;248(22):2095-2108. doi: 10.1177/15353702231209419. Epub 2023 Dec 6. Exp Biol Med (Maywood). 2023. PMID: 38057990 Free PMC article. Review.
-
Pannexin 1 Regulates Network Ensembles and Dendritic Spine Development in Cortical Neurons.eNeuro. 2019 Jun 6;6(3):ENEURO.0503-18.2019. doi: 10.1523/ENEURO.0503-18.2019. Print 2019 May/Jun. eNeuro. 2019. PMID: 31118206 Free PMC article.
-
GABA system as the cause and effect in early development.Neurosci Biobehav Rev. 2024 Jun;161:105651. doi: 10.1016/j.neubiorev.2024.105651. Epub 2024 Apr 4. Neurosci Biobehav Rev. 2024. PMID: 38579901 Review.
-
SNARE protein SNAP25 regulates the chloride-transporter KCC2 in neurons.iScience. 2024 Oct 11;27(11):111156. doi: 10.1016/j.isci.2024.111156. eCollection 2024 Nov 15. iScience. 2024. PMID: 39507243 Free PMC article.
References
-
- Awad PN, Sanon NT, Chattopadhyaya B, Carrico JN, Ouardouz M, Gagne J, Duss S, Wolf D, Desgent S, Cancedda L, et al. . 2016. Reducing premature KCC2 expression rescues seizure susceptibility and spine morphology in atypical febrile seizures. Neurobiol Dis. 91:10–20. - PubMed
-
- Baldi R, Varga C, Tamas G. 2010. Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur J Neurosci. 32:1319–1325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

