Grxcr2 is required for stereocilia morphogenesis in the cochlea
- PMID: 30157177
- PMCID: PMC6114524
- DOI: 10.1371/journal.pone.0201713
Grxcr2 is required for stereocilia morphogenesis in the cochlea
Abstract
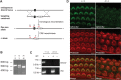
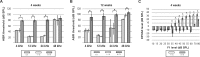
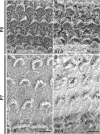
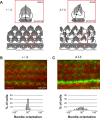
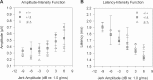
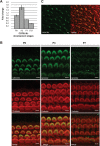
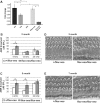
Hearing and balance depend upon the precise morphogenesis and mechanosensory function of stereocilia, the specialized structures on the apical surface of sensory hair cells in the inner ear. Previous studies of Grxcr1 mutant mice indicated a critical role for this gene in control of stereocilia dimensions during development. In this study, we analyzed expression of the paralog Grxcr2 in the mouse and evaluated auditory and vestibular function of strains carrying targeted mutations of the gene. Peak expression of Grxcr2 occurs during early postnatal development of the inner ear and GRXCR2 is localized to stereocilia in both the cochlea and in vestibular organs. Homozygous Grxcr2 deletion mutants exhibit significant hearing loss by 3 weeks of age that is associated with developmental defects in stereocilia bundle orientation and organization. Despite these bundle defects, the mechanotransduction apparatus assembles in relatively normal fashion as determined by whole cell electrophysiological evaluation and FM1-43 uptake. Although Grxcr2 mutants do not exhibit overt vestibular dysfunction, evaluation of vestibular evoked potentials revealed subtle defects of the mutants in response to linear accelerations. In addition, reduced Grxcr2 expression in a hypomorphic mutant strain is associated with progressive hearing loss and bundle defects. The stereocilia localization of GRXCR2, together with the bundle pathologies observed in the mutants, indicate that GRXCR2 plays an intrinsic role in bundle orientation, organization, and sensory function in the inner ear during development and at maturity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Murine GRXCR1 Has a Different Function Than GRXCR2 in the Morphogenesis of Stereocilia.Front Cell Neurosci. 2021 Jul 21;15:714070. doi: 10.3389/fncel.2021.714070. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34366792 Free PMC article.
-
GRXCR2 Regulates Taperin Localization Critical for Stereocilia Morphology and Hearing.Cell Rep. 2018 Oct 30;25(5):1268-1280.e4. doi: 10.1016/j.celrep.2018.09.063. Cell Rep. 2018. PMID: 30380417 Free PMC article.
-
Mutations in Grxcr1 are the basis for inner ear dysfunction in the pirouette mouse.Am J Hum Genet. 2010 Feb 12;86(2):148-60. doi: 10.1016/j.ajhg.2010.01.016. Epub 2010 Feb 4. Am J Hum Genet. 2010. PMID: 20137774 Free PMC article.
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
-
A balance of form and function: planar polarity and development of the vestibular maculae.Semin Cell Dev Biol. 2013 May;24(5):490-8. doi: 10.1016/j.semcdb.2013.03.001. Epub 2013 Mar 15. Semin Cell Dev Biol. 2013. PMID: 23507521 Free PMC article. Review.
Cited by
-
OHC-TRECK: A Novel System Using a Mouse Model for Investigation of the Molecular Mechanisms Associated with Outer Hair Cell Death in the Inner Ear.Sci Rep. 2019 Mar 27;9(1):5285. doi: 10.1038/s41598-019-41711-2. Sci Rep. 2019. PMID: 30918314 Free PMC article.
-
Murine GRXCR1 Has a Different Function Than GRXCR2 in the Morphogenesis of Stereocilia.Front Cell Neurosci. 2021 Jul 21;15:714070. doi: 10.3389/fncel.2021.714070. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34366792 Free PMC article.
-
N-Terminus of GRXCR2 Interacts With CLIC5 and Is Essential for Auditory Perception.Front Cell Dev Biol. 2021 May 5;9:671364. doi: 10.3389/fcell.2021.671364. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34026762 Free PMC article.
-
Grxcr1 regulates hair bundle morphogenesis and is required for normal mechanoelectrical transduction in mouse cochlear hair cells.PLoS One. 2022 Mar 2;17(3):e0261530. doi: 10.1371/journal.pone.0261530. eCollection 2022. PLoS One. 2022. PMID: 35235570 Free PMC article.
-
The actin cytoskeleton in hair bundle development and hearing loss.Hear Res. 2023 Sep 1;436:108817. doi: 10.1016/j.heares.2023.108817. Epub 2023 May 26. Hear Res. 2023. PMID: 37300948 Free PMC article. Review.
References
-
- Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol Suppl. 1985;422:1–69. . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

