Memory Inflation Drives Tissue-Resident Memory CD8+ T Cell Maintenance in the Lung After Intranasal Vaccination With Murine Cytomegalovirus
- PMID: 30154789
- PMCID: PMC6102355
- DOI: 10.3389/fimmu.2018.01861
Memory Inflation Drives Tissue-Resident Memory CD8+ T Cell Maintenance in the Lung After Intranasal Vaccination With Murine Cytomegalovirus
Abstract
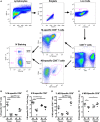
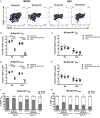
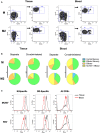
Tissue-resident memory T (TRM) cells provide first-line defense against invading pathogens encountered at barrier sites. In the lungs, TRM cells protect against respiratory infections, but wane more quickly than TRM cells in other tissues. This lack of a sustained TRM population in the lung parenchyma explains, at least in part, why infections with some pathogens, such as influenza virus and respiratory syncytial virus (RSV), recur throughout life. Intranasal (IN) vaccination with a murine cytomegalovirus (MCMV) vector expressing the M protein of RSV (MCMV-M) has been shown to elicit robust populations of CD8+ TRM cells that accumulate over time and mediate early viral clearance. To extend this finding, we compared the inflationary CD8+ T cell population elicited by MCMV-M vaccination with a conventional CD8+ T cell population elicited by an MCMV vector expressing the M2 protein of RSV (MCMV-M2). Vaccination with MCMV-M2 induced a population of M2-specific CD8+ TRM cells that waned rapidly, akin to the M2-specific CD8+ TRM cell population elicited by infection with RSV. In contrast to the natural immunodominance profile, however, coadministration of MCMV-M and MCMV-M2 did not suppress the M-specific CD8+ T cell response, suggesting that progressive expansion was driven by continuous antigen presentation, irrespective of the competitive or regulatory effects of M2-specific CD8+ T cells. Moreover, effective viral clearance mediated by M-specific CD8+ TRM cells was not affected by the coinduction of M2-specific CD8+ T cells. These data show that memory inflation is required for the maintenance of CD8+ TRM cells in the lungs after IN vaccination with MCMV.
Keywords: CD8+ T cells; cytomegalovirus; memory inflation; respiratory syncytial virus; tissue-resident memory; vaccine.
Figures



Similar articles
-
Investigating the Dynamics of MCMV-Specific CD8+ T Cell Responses in Individual Hosts.Front Immunol. 2019 Jun 19;10:1358. doi: 10.3389/fimmu.2019.01358. eCollection 2019. Front Immunol. 2019. PMID: 31281313 Free PMC article.
-
Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung.Mucosal Immunol. 2017 Mar;10(2):545-554. doi: 10.1038/mi.2016.48. Epub 2016 May 25. Mucosal Immunol. 2017. PMID: 27220815 Free PMC article.
-
Viral interference with antigen presentation does not alter acute or chronic CD8 T cell immunodominance in murine cytomegalovirus infection.J Immunol. 2007 Jun 1;178(11):7235-41. doi: 10.4049/jimmunol.178.11.7235. J Immunol. 2007. PMID: 17513772
-
Persistence in Temporary Lung Niches: A Survival Strategy of Lung-Resident Memory CD8+ T Cells.Viral Immunol. 2017 Jul/Aug;30(6):438-450. doi: 10.1089/vim.2017.0016. Epub 2017 Apr 18. Viral Immunol. 2017. PMID: 28418771 Free PMC article. Review.
-
Memory CD8 T cell inflation vs tissue-resident memory T cells: Same patrollers, same controllers?Immunol Rev. 2018 May;283(1):161-175. doi: 10.1111/imr.12649. Immunol Rev. 2018. PMID: 29664565 Review.
Cited by
-
Exploring the Potential of Cytomegalovirus-Based Vectors: A Review.Viruses. 2023 Oct 2;15(10):2043. doi: 10.3390/v15102043. Viruses. 2023. PMID: 37896820 Free PMC article. Review.
-
Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA.Nat Biomed Eng. 2023 Sep 7. doi: 10.1038/s41551-023-01082-6. Online ahead of print. Nat Biomed Eng. 2023. PMID: 37679571
-
Memory T Cells in the Immunoprevention of Cancer: A Switch from Therapeutic to Prophylactic Approaches.J Immunol. 2023 Sep 15;211(6):907-916. doi: 10.4049/jimmunol.2300049. J Immunol. 2023. PMID: 37669503 Free PMC article. Review.
-
Sepsis-induced changes in differentiation, maintenance, and function of memory CD8 T cell subsets.Front Immunol. 2023 Jan 23;14:1130009. doi: 10.3389/fimmu.2023.1130009. eCollection 2023. Front Immunol. 2023. PMID: 36756117 Free PMC article. Review.
-
Pulmonary resident memory T cells in respiratory virus infection and their inspiration on therapeutic strategies.Front Immunol. 2022 Aug 12;13:943331. doi: 10.3389/fimmu.2022.943331. eCollection 2022. Front Immunol. 2022. PMID: 36032142 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

