Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance
- PMID: 30148542
- PMCID: PMC6513199
- DOI: 10.1002/14651858.CD012768.pub2
Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance
Update in
-
Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD012768. doi: 10.1002/14651858.CD012768.pub3. Cochrane Database Syst Rev. 2021. PMID: 33448348 Free PMC article.
Abstract
Background: Tuberculosis (TB) is the world's leading infectious cause of death. Extrapulmonary TB accounts for 15% of TB cases, but the proportion is increasing, and over half a million people were newly diagnosed with rifampicin-resistant TB in 2016. Xpert® MTB/RIF (Xpert) is a World Health Organization (WHO)-recommended, rapid, automated, nucleic acid amplification assay that is used widely for simultaneous detection of Mycobacterium tuberculosis complex and rifampicin resistance in sputum specimens. This Cochrane Review assessed the accuracy of Xpert in extrapulmonary specimens.
Objectives: To determine the diagnostic accuracy of Xpert a) for extrapulmonary TB by site of disease in people presumed to have extrapulmonary TB; and b) for rifampicin resistance in people presumed to have extrapulmonary TB.
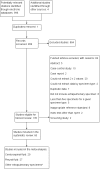
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, Science Citation Index, Web of Science, Latin American Caribbean Health Sciences Literature (LILACS), Scopus, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform, the International Standard Randomized Controlled Trial Number (ISRCTN) Registry, and ProQuest up to 7 August 2017 without language restriction.
Selection criteria: We included diagnostic accuracy studies of Xpert in people presumed to have extrapulmonary TB. We included TB meningitis and pleural, lymph node, bone or joint, genitourinary, peritoneal, pericardial, and disseminated TB. We used culture as the reference standard. For pleural TB, we also included a composite reference standard, which defined a positive result as the presence of granulomatous inflammation or a positive culture result. For rifampicin resistance, we used culture-based drug susceptibility testing or MTBDRplus as the reference standard.
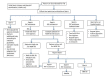
Data collection and analysis: Two review authors independently extracted data, assessed risk of bias and applicability using the QUADAS-2 tool. We determined pooled predicted sensitivity and specificity for TB, grouped by type of extrapulmonary specimen, and for rifampicin resistance. For TB detection, we used a bivariate random-effects model. Recognizing that use of culture may lead to misclassification of cases of extrapulmonary TB as 'not TB' owing to the paucibacillary nature of the disease, we adjusted accuracy estimates by applying a latent class meta-analysis model. For rifampicin resistance detection, we performed univariate meta-analyses for sensitivity and specificity separately to include studies in which no rifampicin resistance was detected. We used theoretical populations with an assumed prevalence to provide illustrative numbers of patients with false positive and false negative results.
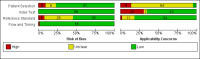
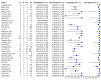
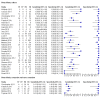
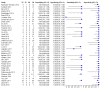
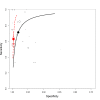
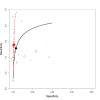
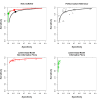
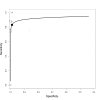
Main results: We included 66 unique studies that evaluated 16,213 specimens for detection of extrapulmonary TB and rifampicin resistance. We identified only one study that evaluated the newest test version, Xpert MTB/RIF Ultra (Ultra), for TB meningitis. Fifty studies (76%) took place in low- or middle-income countries. Risk of bias was low for patient selection, index test, and flow and timing domains and was high or unclear for the reference standard domain (most of these studies decontaminated sterile specimens before culture inoculation). Regarding applicability, in the patient selection domain, we scored high or unclear concern for most studies because either patients were evaluated exclusively as inpatients at tertiary care centres, or we were not sure about the clinical settings.Pooled Xpert sensitivity (defined by culture) varied across different types of specimens (31% in pleural tissue to 97% in bone or joint fluid); Xpert sensitivity was > 80% in urine and bone or joint fluid and tissue. Pooled Xpert specificity (defined by culture) varied less than sensitivity (82% in bone or joint tissue to 99% in pleural fluid and urine). Xpert specificity was ≥ 98% in cerebrospinal fluid, pleural fluid, urine, and peritoneal fluid.Xpert testing in cerebrospinal fluidXpert pooled sensitivity and specificity (95% credible interval (CrI)) against culture were 71.1% (60.9% to 80.4%) and 98.0% (97.0% to 98.8%), respectively (29 studies, 3774 specimens; moderate-certainty evidence).For a population of 1000 people where 100 have TB meningitis on culture, 89 would be Xpert-positive: of these, 18 (20%) would not have TB (false-positives); and 911 would be Xpert-negative: of these, 29 (3%) would have TB (false-negatives).For TB meningitis, ultra sensitivity and specificity against culture (95% confidence interval (CI)) were 90% (55% to 100%) and 90% (83% to 95%), respectively (one study, 129 participants).Xpert testing in pleural fluidXpert pooled sensitivity and specificity (95% CrI) against culture were 50.9% (39.7% to 62.8%) and 99.2% (98.2% to 99.7%), respectively (27 studies, 4006 specimens; low-certainty evidence).For a population of 1000 people where 150 have pleural TB on culture, 83 would be Xpert-positive: of these, seven (8%) would not have TB (false-positives); and 917 would be Xpert-negative: of these, 74 (8%) would have TB (false-negatives).Xpert testing in urineXpert pooled sensitivity and specificity (95% CrI) against culture were 82.7% (69.6% to 91.1%) and 98.7% (94.8% to 99.7%), respectively (13 studies, 1199 specimens; moderate-certainty evidence).For a population of 1000 people where 70 have genitourinary TB on culture, 70 would be Xpert-positive: of these, 12 (17%) would not have TB (false-positives); and 930 would be Xpert-negative: of these, 12 (1%) would have TB (false-negatives).Xpert testing for rifampicin resistanceXpert pooled sensitivity (20 studies, 148 specimens) and specificity (39 studies, 1088 specimens) were 95.0% (89.7% to 97.9%) and 98.7% (97.8% to 99.4%), respectively (high-certainty evidence).For a population of 1000 people where 120 have rifampicin-resistant TB, 125 would be positive for rifampicin-resistant TB: of these, 11 (9%) would not have rifampicin resistance (false-positives); and 875 would be negative for rifampicin-resistant TB: of these, 6 (1%) would have rifampicin resistance (false-negatives).For lymph node TB, the accuracy of culture, the reference standard used, presented a greater concern for bias than in other forms of extrapulmonary TB.
Authors' conclusions: In people presumed to have extrapulmonary TB, Xpert may be helpful in confirming the diagnosis. Xpert sensitivity varies across different extrapulmonary specimens, while for most specimens, specificity is high, the test rarely yielding a positive result for people without TB (defined by culture). Xpert is accurate for detection of rifampicin resistance. For people with presumed TB meningitis, treatment should be based on clinical judgement, and not withheld solely on an Xpert result, as is common practice when culture results are negative.
Conflict of interest statement
We have no financial involvement with any organization or entity that has a financial interest in, or financial conflict with, the subject matter or materials discussed in the review apart from those disclosed.
CMD and SGS work for FIND. FIND is a non‐for‐profit foundation whose mission is to find diagnostic solutions to overcome diseases of poverty in low‐ and middle‐income countries. FIND works closely with the private and public sectors and receives funding from donors and some of its industry partners. FIND has an independent Scientific Advisory Committee and organizational firewalls that protect it against any undue influences in its work or in publication of its findings. More information on FIND’s policy and guidelines for working with private sector partners can be found at
KRS received financial support for the submitted work from the CIDG, and has received financial support for the preparation of systematic reviews and educational materials, consultancy fees from FIND (for the preparation of a systematic review), honoraria, and travel support to attend WHO guideline meetings.
ND received funding from the CIDG.
Figures




















Similar articles
-
Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD012768. doi: 10.1002/14651858.CD012768.pub3. Cochrane Database Syst Rev. 2021. PMID: 33448348 Free PMC article.
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Sep 6;9:CD013359. doi: 10.1002/14651858.CD013359.pub3. PMID: 32853411 Free PMC article. Updated.
-
Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2014 Jan 21;2014(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Jun 07;6:CD009593. doi: 10.1002/14651858.CD009593.pub4. PMID: 24448973 Free PMC article. Updated. Review.
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2022 Sep 6;9(9):CD013359. doi: 10.1002/14651858.CD013359.pub3. Cochrane Database Syst Rev. 2022. PMID: 36065889 Free PMC article. Review.
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2019 Jun 7;6(6):CD009593. doi: 10.1002/14651858.CD009593.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Feb 22;2:CD009593. doi: 10.1002/14651858.CD009593.pub5. PMID: 31173647 Free PMC article. Updated.
Cited by
-
Evaluation of GeneXpert MTB/Rif Ultra assay performance on formalin-fixed paraffin-embedded tissues for Mycobacterium tuberculosis detection.J Med Microbiol. 2024 Oct;73(10):001918. doi: 10.1099/jmm.0.001918. J Med Microbiol. 2024. PMID: 39436387 Free PMC article.
-
Xpert MTB/RIF assay for diagnosis of extrapulmonary tuberculosis in children: a systematic review and meta-analysis.BMC Infect Dis. 2020 Jan 6;20(1):14. doi: 10.1186/s12879-019-4745-1. BMC Infect Dis. 2020. PMID: 31906996 Free PMC article.
-
GeneXpert MTB/RIF Ultra vs Unstimulated Interferon γ (IRISA-TB) for the Diagnosis of Tuberculous Pericarditis in a Tuberculosis-Endemic Setting.Open Forum Infect Dis. 2024 Mar 20;11(3):ofae021. doi: 10.1093/ofid/ofae021. eCollection 2024 Mar. Open Forum Infect Dis. 2024. PMID: 38510916 Free PMC article.
-
Comparison of Performances of GeneXpert MTB/RIF, Bactec MGIT 960, and Bactec Myco/F Systems in Detecting Mycobacterium tuberculosis in Biopsy Tissues: a Retrospective Study.Microbiol Spectr. 2023 Jun 15;11(3):e0141422. doi: 10.1128/spectrum.01414-22. Epub 2023 May 8. Microbiol Spectr. 2023. PMID: 37154704 Free PMC article.
-
Effectiveness and trend forecasting of tuberculosis diagnosis after the introduction of GeneXpert in a city in south-eastern Brazil.PLoS One. 2021 May 28;16(5):e0252375. doi: 10.1371/journal.pone.0252375. eCollection 2021. PLoS One. 2021. PMID: 34048490 Free PMC article.
References
References to studies included in this review
-
- Ablanedo‐Terrazas Y, Alvarado‐de la Barrera C, Hernandez‐Juan R, Ruiz‐Cruz M, Reyes‐Teran G. Xpert MTB/RIF for diagnosis of tuberculous cervical lymphadenitis in HIV‐infected patients. Laryngoscope 2014;124(6):1382‐5. - PubMed
-
- Al‐Ateah SM, Al‐Dowaidi MM, El‐Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non‐respiratory clinical specimens using the Cepheid Gene Xpert(R) system. Saudi Medical Journal 2012;33(10):1100‐5. - PubMed
-
- Arockiaraj J, Michael JS, Amritanand R, David KS, Krishnan V. The role of Xpert MTB/RIF assay in the diagnosis of tubercular spondylodiscitis. European Spine Journal 2017;26(12):3162‐9. - PubMed
References to studies excluded from this review
-
- Alvarez‐Uria G, Azcona JM, Midde M, Naik PK, Reddy S, Reddy R. Rapid diagnosis of pulmonary and extrapulmonary tuberculosis in HIV‐infected patients. Comparison of LED fluorescent microscopy and the GeneXpert MTB/RIF assay in a district hospital in India. Tuberculosis Research and Treatment 2012;2012:932862. [DOI: 10.1155/2012/932862] - DOI - PMC - PubMed
-
- Andrey DO, Hinrikson H, Renzi G, Hibbs J, Adler D, Schrenzel J. Xpert((R)) MTB/RIF assay sensitivity with different methods of CSF processing for the diagnosis of TB meningitis. International Journal of Tuberculosis and Lung Disease 2015;19(12):1555‐6. - PubMed
-
- Arockiaraj J, Amritanand R, Krishnan V, Sundararaj G, Michael J. GeneXpert polymerase chain reaction (PCR) Test: role in the diagnosis of tubercular spondylodiscitis. Spine Journal 2015;15(10 Suppl 1):200S‐201S.
Additional references
-
- American Thoracic Society, the Centers for Disease Control and Prevention, Infectious Disease Society of America. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. American Journal Respiratory and Critical Care Medicine 2000;161(4 Pt 1):1376‐95. - PubMed
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401–6. - PubMed
-
- Begg CB, Greenes RA. Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics 1983;39(1):207‐15. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

