Synthesis, pharmacology and preclinical evaluation of 11C-labeled 1,3-dihydro-2H-benzo[d]imidazole-2-ones for imaging γ8-dependent transmembrane AMPA receptor regulatory protein
- PMID: 30145376
- PMCID: PMC6245653
- DOI: 10.1016/j.ejmech.2018.08.019
Synthesis, pharmacology and preclinical evaluation of 11C-labeled 1,3-dihydro-2H-benzo[d]imidazole-2-ones for imaging γ8-dependent transmembrane AMPA receptor regulatory protein
Abstract
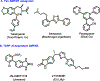
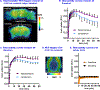
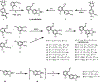
a-Amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) receptors are implicated in the pathology of neurological diseases such as epilepsy and schizophrenia. As pan antagonists for this target are often accompanied with undesired effects at high doses, one of the recent drug discovery approaches has shifted to subtype-selective AMPA receptor (AMPAR) antagonists, specifically, via modulating transmembrane AMPAR regulatory proteins (TARPs). The quantification of AMPARs by positron emission tomography (PET) would help obtain insights into disease conditions in the living brain and advance the translational development of AMPAR antagonists. Herein we report the design, synthesis and preclinical evaluation of a series of TARP γ-8 antagonists, amenable for radiolabeling, for the development of subtype-selective AMPAR PET imaging agents. Based on the pharmacology evaluation, molecular docking studies and physiochemical properties, we have identified several promising lead compounds 3, 17-19 and 21 for in vivo PET studies. All candidate compounds were labeled with [11C]COCl2 in high radiochemical yields (13-31% RCY) and high molar activities (35-196 GBq/μmol). While tracers 30 ([11C]17) &32 ([11C]21) crossed the blood-brain barrier and showed heterogeneous distribution in PET studies, consistent with TARP γ-8 expression, high nonspecific binding prevented further evaluation. To our delight, tracer 31 ([11C]3) showed good in vitro specific binding and characteristic high uptake in the hippocampus in rat brain tissues, which provides the guideline for further development of a new generation subtype selective TARP γ-8 dependent AMPAR tracers.
Keywords: AMPA; Epilepsy; Ionotropic glutamate receptor; Positron emission tomography; TARP; Transmembrane AMPA receptor regulatory protein.
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
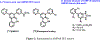
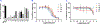
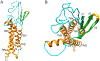
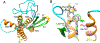

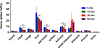
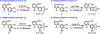
Similar articles
-
Characterizing the binding and function of TARP γ8-selective AMPA receptor modulators.J Biol Chem. 2020 Oct 23;295(43):14565-14577. doi: 10.1074/jbc.RA120.014135. Epub 2020 Aug 3. J Biol Chem. 2020. PMID: 32747446 Free PMC article.
-
Influence of the TARP γ8-Selective Negative Allosteric Modulator JNJ-55511118 on AMPA Receptor Gating and Channel Conductance.Mol Pharmacol. 2022 May;101(5):343-356. doi: 10.1124/molpharm.121.000473. Epub 2022 Mar 3. Mol Pharmacol. 2022. PMID: 35246481 Free PMC article.
-
Preclinical Evaluation of Azabenzimidazole-Based PET Radioligands for γ-8 Dependent Transmembrane AMPA Receptor Regulatory Protein Imaging.Chembiochem. 2024 Mar 15;25(6):e202300813. doi: 10.1002/cbic.202300813. Epub 2024 Feb 19. Chembiochem. 2024. PMID: 38227784
-
Positron Emission Tomography (PET) Ligand Development for Ionotropic Glutamate Receptors: Challenges and Opportunities for Radiotracer Targeting N-Methyl-d-aspartate (NMDA), α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA), and Kainate Receptors.J Med Chem. 2019 Jan 24;62(2):403-419. doi: 10.1021/acs.jmedchem.8b00714. Epub 2018 Aug 27. J Med Chem. 2019. PMID: 30110164 Free PMC article. Review.
-
Structural and functional insights into transmembrane AMPA receptor regulatory protein complexes.J Gen Physiol. 2019 Dec 2;151(12):1347-1356. doi: 10.1085/jgp.201812264. Epub 2019 Oct 15. J Gen Physiol. 2019. PMID: 31615831 Free PMC article. Review.
Cited by
-
Enhanced TARP-γ8-PSD-95 coupling in excitatory neurons contributes to the rapid antidepressant-like action of ketamine in male mice.Nat Commun. 2023 Dec 2;14(1):7971. doi: 10.1038/s41467-023-42780-8. Nat Commun. 2023. PMID: 38042894 Free PMC article.
-
Novel Reversible-Binding PET Ligands for Imaging Monoacylglycerol Lipase Based on the Piperazinyl Azetidine Scaffold.J Med Chem. 2021 Oct 14;64(19):14283-14298. doi: 10.1021/acs.jmedchem.1c00747. Epub 2021 Sep 27. J Med Chem. 2021. PMID: 34569803 Free PMC article.
-
Design, Synthesis, and Evaluation of Reversible and Irreversible Monoacylglycerol Lipase Positron Emission Tomography (PET) Tracers Using a "Tail Switching" Strategy on a Piperazinyl Azetidine Skeleton.J Med Chem. 2019 Apr 11;62(7):3336-3353. doi: 10.1021/acs.jmedchem.8b01778. Epub 2019 Mar 21. J Med Chem. 2019. PMID: 30829483 Free PMC article.
-
Preclinical Evaluation of Novel Positron Emission Tomography (PET) Probes for Imaging Leucine-Rich Repeat Kinase 2 (LRRK2).J Med Chem. 2024 Feb 22;67(4):2559-2569. doi: 10.1021/acs.jmedchem.3c01687. Epub 2024 Feb 2. J Med Chem. 2024. PMID: 38305157 Free PMC article.
-
Imaging of Transmembrane AMPA Receptor Regulatory Proteins by Positron Emission Tomography.J Med Chem. 2022 Jul 14;65(13):9144-9158. doi: 10.1021/acs.jmedchem.2c00377. Epub 2022 Jun 28. J Med Chem. 2022. PMID: 35762919 Free PMC article.
References
-
- Meldrum BS, Glutamate as a neurotransmitter in the brain: review of physiology and pathology, J. Nutr, 130 (2000) 1007S–1015S. - PubMed
-
- Dingledine R, Borges K, Bowie D, Traynelis SF, The glutamate receptor ion channels, Pharmacol. Rev, 51 (1999) 7–61. - PubMed
-
- Mayer ML, Armstrong N, Structure and function of glutamate receptor ion channels, Annu. Rev. Physiol, 66 (2004) 161–181. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

