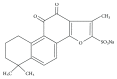
Sodium Tanshinone IIA Sulfonate Prevents Angiotensin II-Induced Differentiation of Human Atrial Fibroblasts into Myofibroblasts
- PMID: 30140368
- PMCID: PMC6081515
- DOI: 10.1155/2018/6712585
Sodium Tanshinone IIA Sulfonate Prevents Angiotensin II-Induced Differentiation of Human Atrial Fibroblasts into Myofibroblasts
Abstract
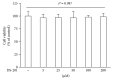
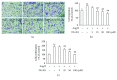
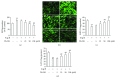
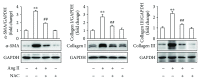
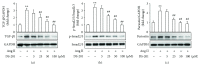
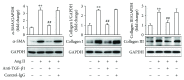
Differentiation of atrial fibroblasts into myofibroblasts plays a critical role in atrial fibrosis. Sodium tanshinone IIA sulfonate (DS-201), a water-soluble derivative of tanshinone IIA, has been shown to have potent antifibrotic properties. However, the protective effects of DS-201 on angiotensin II- (Ang II-) induced differentiation of atrial fibroblasts into myofibroblasts remain to be elucidated. In this study, human atrial fibroblasts were stimulated with Ang II in the presence or absence of DS-201. Then, α-smooth muscle actin (α-SMA), collagen I, and collagen III expression and reactive oxygen species (ROS) generation were measured. The expression of transforming growth factor-β1 (TGF-β1) and the downstream signaling of TGF-β1, such as phosphorylation of Smad2/3, were also determined. The results demonstrated that DS-201 significantly prevented Ang II-induced human atrial fibroblast migration and decreased Ang II-induced α-SMA, collagen I, and collagen III expression. Furthermore, increased production of ROS and expression of TGF-β1 stimulated by Ang II were also significantly inhibited by DS-201. Consistent with these results, DS-201 significantly inhibited Ang II-evoked Smad2/3 phosphorylation and periostin expression. These results and the experiments involving N-acetyl cysteine (antioxidant) and an anti-TGF-β1 antibody suggest that DS-201 prevent Ang II-induced differentiation of atrial fibroblasts to myofibroblasts, at least in part, through suppressing oxidative stress and inhibiting the activation of TGF-β1 signaling pathway. All of these data indicate the potential utility of DS-201 for the treatment of cardiac fibrosis.
Figures

Similar articles
-
Sodium tanshinone IIA sulfonate attenuates the transforming growth factor-β1-induced differentiation of atrial fibroblasts into myofibroblasts in vitro.Int J Mol Med. 2015 Apr;35(4):1026-32. doi: 10.3892/ijmm.2015.2087. Epub 2015 Feb 2. Int J Mol Med. 2015. PMID: 25647570
-
FOXF1 ameliorates angiotensin II-induced cardiac fibrosis in cardiac fibroblasts through inhibiting the TGF-β1/Smad3 signaling pathway.J Recept Signal Transduct Res. 2020 Dec;40(6):493-500. doi: 10.1080/10799893.2020.1772299. Epub 2020 Jun 4. J Recept Signal Transduct Res. 2020. PMID: 32496870
-
Tanshinone IIA inhibits angiotensin II-induced cell proliferation in rat cardiac fibroblasts.Am J Chin Med. 2011;39(2):381-94. doi: 10.1142/S0192415X11008890. Am J Chin Med. 2011. PMID: 21476213
-
Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review.
-
TGF-beta1 and angiotensin networking in cardiac remodeling.Cardiovasc Res. 2004 Aug 15;63(3):423-32. doi: 10.1016/j.cardiores.2004.04.030. Cardiovasc Res. 2004. PMID: 15276467 Review.
Cited by
-
Tanshinones induce tumor cell apoptosis via directly targeting FHIT.Sci Rep. 2021 Jun 9;11(1):12217. doi: 10.1038/s41598-021-91708-z. Sci Rep. 2021. PMID: 34108553 Free PMC article.
-
Research Progress on Natural Products' Therapeutic Effects on Atrial Fibrillation by Regulating Ion Channels.Cardiovasc Ther. 2022 Mar 22;2022:4559809. doi: 10.1155/2022/4559809. eCollection 2022. Cardiovasc Ther. 2022. PMID: 35387267 Free PMC article. Review.
-
Hydroxysafflor yellow A mitigates myocardial fibrosis induced by isoproterenol and angiotensin II.Am J Transl Res. 2022 Dec 15;14(12):8588-8598. eCollection 2022. Am J Transl Res. 2022. PMID: 36628216 Free PMC article.
-
Tanshinone IIA attenuates heart failure via inhibiting oxidative stress in myocardial infarction rats.Mol Med Rep. 2021 Jun;23(6):404. doi: 10.3892/mmr.2021.12043. Epub 2021 Mar 31. Mol Med Rep. 2021. PMID: 33786621 Free PMC article.
-
Upregulation of Transient Receptor Potential Canonical Type 3 Channel via AT1R/TGF-β1/Smad2/3 Induces Atrial Fibrosis in Aging and Spontaneously Hypertensive Rats.Oxid Med Cell Longev. 2019 Nov 23;2019:4025496. doi: 10.1155/2019/4025496. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31871548 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

