Endoplasmic reticulum-to-Golgi transitions upon herpes virus infection
- PMID: 30135710
- PMCID: PMC6080407
- DOI: 10.12688/f1000research.12252.2
Endoplasmic reticulum-to-Golgi transitions upon herpes virus infection
Abstract
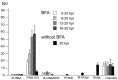
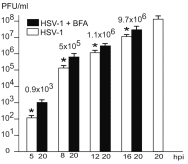
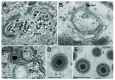
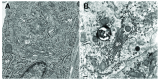
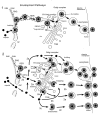
Background: Herpesvirus capsids are assembled in the nucleus, translocated to the perinuclear space by budding, acquiring tegument and envelope, or released to the cytoplasm via impaired nuclear envelope. One model proposes that envelopment, "de-envelopment" and "re-envelopment" is essential for production of infectious virus. Glycoproteins gB/gH were reported to be essential for de-envelopment, by fusion of the "primary" envelope with the outer nuclear membrane. Yet, a high proportion of enveloped virions generated from genomes with deleted gB/gH were found in the cytoplasm and extracellular space, suggesting the existence of alternative exit routes. Methods: We investigated the relatedness between the nuclear envelope and membranes of the endoplasmic reticulum and Golgi complex, in cells infected with either herpes simplex virus 1 (HSV-1) or a Us3 deletion mutant thereof, or with bovine herpesvirus 1 (BoHV-1) by transmission and scanning electron microscopy, employing freezing technique protocols. Results: The Golgi complex is a compact entity in a juxtanuclear position covered by a membrane on the cis face. Golgi membranes merge with membranes of the endoplasmic reticulum forming an entity with the perinuclear space. All compartments contained enveloped virions. After treatment with brefeldin A, HSV-1 virions aggregated in the perinuclear space and endoplasmic reticulum, while infectious progeny virus was still produced. Conclusions: The data suggest that virions derived by budding at nuclear membranes are intraluminally transported from the perinuclear space via Golgi -endoplasmic reticulum transitions into Golgi cisternae for packaging. Virions derived by budding at nuclear membranes are infective like Us3 deletion mutants, which accumulate in the perinuclear space. Therefore, i) de-envelopment followed by re-envelopment is not essential for production of infective progeny virus, ii) the process taking place at the outer nuclear membrane is budding not fusion, and iii) naked capsids gain access to the cytoplasmic matrix via impaired nuclear envelope as reported earlier.
Keywords: Golgi complex; brefeldin A; egress pathway; endoplasmic reticulum; envelopment; herpes virus; intraluminal transport.
Conflict of interest statement
No competing interests were disclosed.
Figures







Similar articles
-
The herpes simplex virus 1 Us3 kinase is involved in assembly of membranes needed for viral envelopment and in distribution of glycoprotein K.F1000Res. 2019 May 23;8:727. doi: 10.12688/f1000research.19194.1. eCollection 2019. F1000Res. 2019. PMID: 31448105 Free PMC article.
-
Herpes simplex virus 1 envelopment follows two diverse pathways.J Virol. 2005 Oct;79(20):13047-59. doi: 10.1128/JVI.79.20.13047-13059.2005. J Virol. 2005. PMID: 16189007 Free PMC article.
-
Intra-nuclear localization of two envelope proteins, gB and gD, of herpes simplex virus.Arch Virol. 1996;141(3-4):505-24. doi: 10.1007/BF01718314. Arch Virol. 1996. PMID: 8645092
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
-
Intriguing interplay between viral proteins during herpesvirus assembly or: the herpesvirus assembly puzzle.Vet Microbiol. 2006 Mar 31;113(3-4):163-9. doi: 10.1016/j.vetmic.2005.11.040. Epub 2005 Dec 5. Vet Microbiol. 2006. PMID: 16330166 Review.
Cited by
-
Models of Intracellular Transport: Pros and Cons.Front Cell Dev Biol. 2019 Aug 7;7:146. doi: 10.3389/fcell.2019.00146. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31440506 Free PMC article.
-
The herpes simplex virus 1 Us3 kinase is involved in assembly of membranes needed for viral envelopment and in distribution of glycoprotein K.F1000Res. 2019 May 23;8:727. doi: 10.12688/f1000research.19194.1. eCollection 2019. F1000Res. 2019. PMID: 31448105 Free PMC article.
-
Molecular mechanisms of human herpes viruses inferring with host immune surveillance.J Immunother Cancer. 2020 Jul;8(2):e000841. doi: 10.1136/jitc-2020-000841. J Immunother Cancer. 2020. PMID: 32616556 Free PMC article. Review.
-
A bird's-eye view of the endoplasmic reticulum in filamentous fungi.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0002723. doi: 10.1128/mmbr.00027-23. Epub 2024 Feb 19. Microbiol Mol Biol Rev. 2024. PMID: 38372526 Review.
-
Comparison of the Cisterna Maturation-Progression Model with the Kiss-and-Run Model of Intra-Golgi Transport: Role of Cisternal Pores and Cargo Domains.Int J Mol Sci. 2022 Mar 25;23(7):3590. doi: 10.3390/ijms23073590. Int J Mol Sci. 2022. PMID: 35408951 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

