BDNF inhibits neurodegenerative disease-associated asparaginyl endopeptidase activity via phosphorylation by AKT
- PMID: 30135302
- PMCID: PMC6141178
- DOI: 10.1172/jci.insight.99007
BDNF inhibits neurodegenerative disease-associated asparaginyl endopeptidase activity via phosphorylation by AKT
Abstract
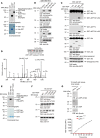
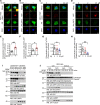
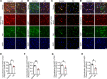
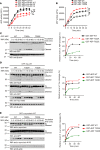
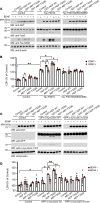
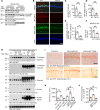
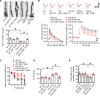
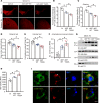
AEP is an age-dependent lysosomal asparaginyl endopeptidase that cleaves numerous substrates including tau and α-synuclein and mediates their pathological roles in neurodegenerative diseases. However, the molecular mechanism regulating this critical protease remains incompletely understood. Here, we show that Akt phosphorylates AEP on residue T322 upon brain-derived neurotrophic factor (BDNF) treatment and triggers its lysosomal translocation and inactivation. When BDNF levels are reduced in neurodegenerative diseases, AEP T322 phosphorylation is attenuated. Consequently, AEP is activated and translocates into the cytoplasm, where it cleaves both tau and α-synuclein. Remarkably, the unphosphorylated T322A mutant increases tau or α-synuclein cleavage by AEP and augments cell death, whereas phosphorylation mimetic T322E mutant represses these effects. Interestingly, viral injection of T322E into Tau P301S mice antagonizes tau N368 cleavage and tau pathologies, rescuing synaptic dysfunction and cognitive deficits. By contrast, viral administration of T322A into young α-SNCA mice elicits α-synuclein N103 cleavage and promotes dopaminergic neuronal loss, facilitating motor defects. Therefore, our findings support the notion that BDNF contributes to the pathogenesis of neurodegenerative diseases by suppressing AEP via Akt phosphorylation.
Keywords: Cell Biology; Molecular pathology; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures

Similar articles
-
Delta-secretase-cleaved Tau antagonizes TrkB neurotrophic signalings, mediating Alzheimer's disease pathologies.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):9094-9102. doi: 10.1073/pnas.1901348116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004063 Free PMC article.
-
Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer's disease.Nat Med. 2014 Nov;20(11):1254-62. doi: 10.1038/nm.3700. Epub 2014 Oct 19. Nat Med. 2014. PMID: 25326800 Free PMC article.
-
Initiation of Parkinson's disease from gut to brain by δ-secretase.Cell Res. 2020 Jan;30(1):70-87. doi: 10.1038/s41422-019-0241-9. Epub 2019 Oct 24. Cell Res. 2020. PMID: 31649329 Free PMC article.
-
Asparagine endopeptidase is an innovative therapeutic target for neurodegenerative diseases.Expert Opin Ther Targets. 2016 Oct;20(10):1237-45. doi: 10.1080/14728222.2016.1182990. Epub 2016 May 13. Expert Opin Ther Targets. 2016. PMID: 27115710 Free PMC article. Review.
-
Following the leader: fibrillization of alpha-synuclein and tau.Exp Neurol. 2004 Jun;187(2):235-9. doi: 10.1016/j.expneurol.2004.02.008. Exp Neurol. 2004. PMID: 15144849 Review. No abstract available.
Cited by
-
Treating Parkinson's Disease via Activation of BDNF/TrkB Signaling Pathways and Inhibition of Delta-Secretase.Neurotherapeutics. 2022 Jul;19(4):1283-1297. doi: 10.1007/s13311-022-01248-1. Epub 2022 May 20. Neurotherapeutics. 2022. PMID: 35595958 Free PMC article.
-
Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating's Effects on Human Health.Nutrients. 2020 Dec 8;12(12):3770. doi: 10.3390/nu12123770. Nutrients. 2020. PMID: 33302500 Free PMC article.
-
C/EBPβ/AEP Signaling Drives Alzheimer's Disease Pathogenesis.Neurosci Bull. 2023 Jul;39(7):1173-1185. doi: 10.1007/s12264-023-01025-w. Epub 2023 Feb 3. Neurosci Bull. 2023. PMID: 36735152 Free PMC article. Review.
-
Delta-secretase-cleaved Tau antagonizes TrkB neurotrophic signalings, mediating Alzheimer's disease pathologies.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):9094-9102. doi: 10.1073/pnas.1901348116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004063 Free PMC article.
-
δ-secretase in neurodegenerative diseases: mechanisms, regulators and therapeutic opportunities.Transl Neurodegener. 2020 Jan 6;9:1. doi: 10.1186/s40035-019-0179-3. eCollection 2020. Transl Neurodegener. 2020. PMID: 31911834 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

