Cholinergic Nerve Differentiation of Mesenchymal Stem Cells Derived from Long-Term Cryopreserved Human Dental Pulp In Vitro and Analysis of Their Motor Nerve Regeneration Potential In Vivo
- PMID: 30126144
- PMCID: PMC6122009
- DOI: 10.3390/ijms19082434
Cholinergic Nerve Differentiation of Mesenchymal Stem Cells Derived from Long-Term Cryopreserved Human Dental Pulp In Vitro and Analysis of Their Motor Nerve Regeneration Potential In Vivo
Abstract
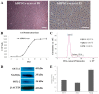
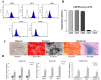
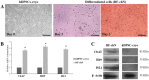
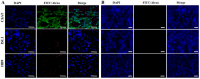
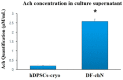
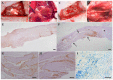
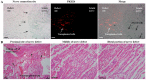
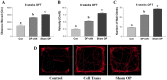
The reduction of choline acetyltransferase, caused by the loss of cholinergic neurons, leads to the absence of acetylcholine (Ach), which is related to motor nerve degeneration. The aims of the present study were to evaluate the in vitro cholinergic nerve differentiation potential of mesenchymal stem cells from cryopreserved human dental pulp (hDPSCs-cryo) and to analyze the scale of in vivo motor nerve regeneration. The hDPSCs-cryo were isolated and cultured from cryopreserved dental pulp tissues, and thereafter differentiated into cholinergic neurons using tricyclodecane-9-yl-xanthogenate (D609). Differentiated cholinergic neurons (DF-chN) were transplanted into rats to address sciatic nerve defects, and the scale of in vivo motor nerve regeneration was analyzed. During in vitro differentiation, the cells showed neuron-like morphological changes including axonal fibers and neuron body development, and revealed high expression of cholinergic neuron-specific markers at both the messenger RNA (mRNA) and protein levels. Importantly, DF-chN showed significant Ach secretion ability. At eight weeks after DF-chN transplantation in rats with sciatic nerve defects, notably increased behavioral activities were detected with an open-field test, with enhanced low-affinity nerve growth factor receptor (p75NGFR) expression detected using immunohistochemistry. These results demonstrate that stem cells from cryopreserved dental pulp can successfully differentiate into cholinergic neurons in vitro and enhance motor nerve regeneration when transplanted in vivo. Additionally, this study suggests that long-term preservation of dental pulp tissue is worthwhile for use as an autologous cell resource in the field of nerve regeneration, including cholinergic nerves.
Keywords: cell transplantation; cholinergic nerve differentiation; dental pulp stem cells; tissue cryopreservation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transplantation of Human Dental Pulp-Derived Stem Cells or Differentiated Neuronal Cells from Human Dental Pulp-Derived Stem Cells Identically Enhances Regeneration of the Injured Peripheral Nerve.Stem Cells Dev. 2017 Sep 1;26(17):1247-1257. doi: 10.1089/scd.2017.0068. Epub 2017 Jul 25. Stem Cells Dev. 2017. PMID: 28657463
-
Stem Cells from Cryopreserved Human Dental Pulp Tissues Sequentially Differentiate into Definitive Endoderm and Hepatocyte-Like Cells in vitro.Int J Med Sci. 2017 Nov 2;14(13):1418-1429. doi: 10.7150/ijms.22152. eCollection 2017. Int J Med Sci. 2017. PMID: 29200956 Free PMC article.
-
Cholinergic neuron-like cells derived from bone marrow stromal cells induced by tricyclodecane-9-yl-xanthogenate promote functional recovery and neural protection after spinal cord injury.Cell Transplant. 2013;22(6):961-75. doi: 10.3727/096368912X657413. Epub 2012 Oct 1. Cell Transplant. 2013. PMID: 23031841
-
The Use of Stem Cells in Neural Regeneration: A Review of Current Opinion.Curr Stem Cell Res Ther. 2018;13(7):608-617. doi: 10.2174/1574888X13666180720100738. Curr Stem Cell Res Ther. 2018. PMID: 30027853 Review.
-
Dental Pulp Stem Cells and Neurogenesis.Adv Exp Med Biol. 2018;1083:63-75. doi: 10.1007/5584_2017_71. Adv Exp Med Biol. 2018. PMID: 28687960 Review.
Cited by
-
Synergistic Effect of the Long-Term Overexpression of Bcl-2 and BDNF Lentiviral in Cell Protecting against Death and Generating TH Positive and CHAT Positive Cells from MSC.Int J Mol Sci. 2021 Jun 30;22(13):7086. doi: 10.3390/ijms22137086. Int J Mol Sci. 2021. PMID: 34209365 Free PMC article.
-
SOCS7-Derived BC-Box Motif Peptide Mediated Cholinergic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2023 Feb 1;24(3):2786. doi: 10.3390/ijms24032786. Int J Mol Sci. 2023. PMID: 36769102 Free PMC article.
-
Mesenchymal Stem Cells in Renal Fibrosis: The Flame of Cytotherapy.Stem Cells Int. 2019 Jan 13;2019:8387350. doi: 10.1155/2019/8387350. eCollection 2019. Stem Cells Int. 2019. PMID: 30766607 Free PMC article. Review.
-
Potential of dental pulp stem cells and their products in promoting peripheral nerve regeneration and their future applications.World J Stem Cells. 2023 Oct 26;15(10):960-978. doi: 10.4252/wjsc.v15.i10.960. World J Stem Cells. 2023. PMID: 37970238 Free PMC article. Review.
-
A Two-Stage Process for Differentiation of Wharton's Jelly-Derived Mesenchymal Stem Cells into Neuronal-like Cells.Stem Cells Int. 2021 May 28;2021:6631651. doi: 10.1155/2021/6631651. eCollection 2021. Stem Cells Int. 2021. PMID: 34135973 Free PMC article.
References
-
- Sun C., Shao J., Su L., Zhao J., Bi J., Yang S., Zhang S., Gao J., Miao J. Cholinergic neuron-like cells derived from bone marrow stromal cells induced by tricyclodecane-9-yl-xanthogenate promote functional recovery and neural protection after spinal cord injury. Cell Transplant. 2013;22:961–975. doi: 10.3727/096368912X657413. - DOI - PubMed
-
- Lee J.K., Jin H.K., Endo S., Schuchman E.H., Carter J.E., Bae S.J. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28:329–343. doi: 10.1002/stem.277. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

