Golgin-97 Targets Ectopically Expressed Inward Rectifying Potassium Channel, Kir2.1, to the trans-Golgi Network in COS-7 Cells
- PMID: 30123141
- PMCID: PMC6085455
- DOI: 10.3389/fphys.2018.01070
Golgin-97 Targets Ectopically Expressed Inward Rectifying Potassium Channel, Kir2.1, to the trans-Golgi Network in COS-7 Cells
Abstract
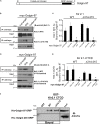
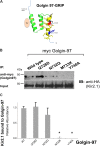
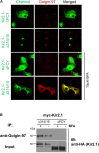
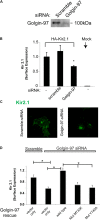
The inward rectifying potassium channel, Kir2.1, is selected as cargo at the trans-Golgi network (TGN) for export to the cell surface through a unique signal-dependent interaction with the AP1 clathrin-adaptor, but it is unknown how the channel is targeted at earlier stages in the secretory pathway for traffic to the TGN. Here we explore a mechanism. A systematic screen of Golgi tethers identified Golgin-97 as a Kir2.1 binding partner. In vitro protein-interaction studies revealed the interaction is direct, occurring between the GRIP domain of Golgin-97 and the cytoplasmic domain of Kir2.1. Imaging and interaction studies in COS-7 cells suggest that Golgi-97 binds to the channel en route through the Golgi. RNA interference-mediated knockdown of Golgin-97 prevented exit of Kir2.1 from the Golgi. These observations identify Golgin-97 as a Kir2.1 binding partner that is required for targeting the channel to the TGN. Based on our studies in COS-7 cells, we propose Golgi-97 facilitates formation of AP1-dependent export carriers for Kir2.1 by coupling anterograde delivery of Kir2.1 with retrograde recycling of AP-1 containing endosomes to the TGN.
Keywords: clathrin; golgi apparatus; inward rectifying K channel; membrane trafficking; potassium channel.
Figures


Comment in
-
Commentary: Golgin-97 Targets Ectopically Expressed Inward Rectifying Potassium Channel, Kir2.1, to the Trans-Golgi Network in COS-7 Cells.Front Physiol. 2018 Oct 5;9:1401. doi: 10.3389/fphys.2018.01401. eCollection 2018. Front Physiol. 2018. PMID: 30344494 Free PMC article. No abstract available.
Similar articles
-
Commentary: Golgin-97 Targets Ectopically Expressed Inward Rectifying Potassium Channel, Kir2.1, to the Trans-Golgi Network in COS-7 Cells.Front Physiol. 2018 Oct 5;9:1401. doi: 10.3389/fphys.2018.01401. eCollection 2018. Front Physiol. 2018. PMID: 30344494 Free PMC article. No abstract available.
-
A Common Signal Patch Drives AP-1 Protein-dependent Golgi Export of Inwardly Rectifying Potassium Channels.J Biol Chem. 2016 Jul 15;291(29):14963-72. doi: 10.1074/jbc.M116.729822. Epub 2016 May 23. J Biol Chem. 2016. PMID: 27226616 Free PMC article.
-
Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure.Cell. 2011 Jun 24;145(7):1102-15. doi: 10.1016/j.cell.2011.06.007. Cell. 2011. PMID: 21703452 Free PMC article.
-
Domains of the TGN: coats, tethers and G proteins.Traffic. 2004 May;5(5):315-26. doi: 10.1111/j.1398-9219.2004.00182.x. Traffic. 2004. PMID: 15086781 Review.
-
Clathrin and post-Golgi trafficking: a very complicated issue.Trends Plant Sci. 2014 Mar;19(3):134-9. doi: 10.1016/j.tplants.2013.10.008. Epub 2013 Nov 19. Trends Plant Sci. 2014. PMID: 24263003 Review.
Cited by
-
The network of cardiac KIR2.1: its function, cellular regulation, electrical signaling, diseases and new drug avenues.Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep;397(9):6369-6389. doi: 10.1007/s00210-024-03116-5. Epub 2024 Apr 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38683369 Free PMC article. Review.
-
Disease Associated Mutations in KIR Proteins Linked to Aberrant Inward Rectifier Channel Trafficking.Biomolecules. 2019 Oct 25;9(11):650. doi: 10.3390/biom9110650. Biomolecules. 2019. PMID: 31731488 Free PMC article. Review.
-
PKD-dependent PARP12-catalyzed mono-ADP-ribosylation of Golgin-97 is required for E-cadherin transport from Golgi to plasma membrane.Proc Natl Acad Sci U S A. 2022 Jan 4;119(1):e2026494119. doi: 10.1073/pnas.2026494119. Proc Natl Acad Sci U S A. 2022. PMID: 34969853 Free PMC article.
-
The Potassium Channel Odyssey: Mechanisms of Traffic and Membrane Arrangement.Int J Mol Sci. 2019 Feb 9;20(3):734. doi: 10.3390/ijms20030734. Int J Mol Sci. 2019. PMID: 30744118 Free PMC article. Review.
-
Unlocking Golgi: Why Does Morphology Matter?Biochemistry (Mosc). 2019 Dec;84(12):1490-1501. doi: 10.1134/S0006297919120083. Biochemistry (Mosc). 2019. PMID: 31870253 Free PMC article. Review.
References
-
- Ballester Y., Benson D. W., Wong B., Law I. H., Mathews K. D., Vanoye C. G., et al. (2006). Trafficking-competent and trafficking-defective KCNJ2 mutations in Andersen syndrome. Hum. Mutat. 27:388. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

