Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson's disease models
- PMID: 30120245
- PMCID: PMC6098059
- DOI: 10.1038/s12276-018-0124-z
Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson's disease models
Abstract
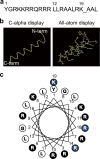
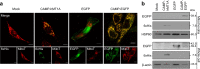
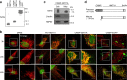
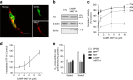
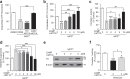
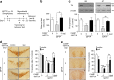
An excess of reactive oxygen species (ROS) relative to the antioxidant capacity causes oxidative stress, which plays a role in the development of Parkinson's disease (PD). Because mitochondria are both sites of ROS generation and targets of ROS damage, the delivery of antioxidants to mitochondria might prevent or alleviate PD. To transduce the antioxidant protein human metallothionein 1A (hMT1A) into mitochondria, we computationally designed a cell-penetrating artificial mitochondria-targeting peptide (CAMP). The recombinant CAMP-conjugated hMT1A fusion protein (CAMP-hMT1A) successfully localized to the mitochondria. Treating a cell culture model of PD with CAMP-hMT1A restored tyrosine hydroxylase expression and mitochondrial activity and reduced ROS production. Furthermore, injection of CAMP-hMT1A into the brain of a mouse model of PD rescued movement impairment and dopaminergic neuronal degeneration. CAMP-hMT1A delivery into mitochondria might be therapeutic against PD by alleviating mitochondrial damage, and we predict that CAMP could be used to deliver other cargo proteins to the mitochondria.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
PEP-1-HO-1 prevents MPTP-induced degeneration of dopaminergic neurons in a Parkinson's disease mouse model.BMB Rep. 2014 Oct;47(10):569-74. doi: 10.5483/bmbrep.2014.47.10.286. BMB Rep. 2014. PMID: 24499676 Free PMC article.
-
Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson's disease.Neuroscience. 2006 Jun 19;140(1):67-76. doi: 10.1016/j.neuroscience.2006.02.007. Epub 2006 Mar 14. Neuroscience. 2006. PMID: 16533572
-
Application of a blood-brain-barrier-penetrating form of GDNF in a mouse model for Parkinson's disease.Brain Res. 2006 Apr 12;1082(1):61-6. doi: 10.1016/j.brainres.2006.01.083. Brain Res. 2006. PMID: 16703672
-
The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson's disease.Gene. 2013 Dec 10;532(1):18-23. doi: 10.1016/j.gene.2013.07.085. Epub 2013 Aug 15. Gene. 2013. PMID: 23954870 Review.
-
Mitochondria as an easy target to oxidative stress events in Parkinson's disease.CNS Neurol Disord Drug Targets. 2012 Jun 1;11(4):430-8. doi: 10.2174/187152712800792875. CNS Neurol Disord Drug Targets. 2012. PMID: 22483310 Review.
Cited by
-
Smart Stimuli-Responsive and Mitochondria Targeting Delivery in Cancer Therapy.Int J Nanomedicine. 2021 Jun 15;16:4117-4146. doi: 10.2147/IJN.S315368. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34163163 Free PMC article. Review.
-
Multifunctional Metallothioneins as a Target for Neuroprotection in Parkinson's Disease.Antioxidants (Basel). 2023 Apr 6;12(4):894. doi: 10.3390/antiox12040894. Antioxidants (Basel). 2023. PMID: 37107269 Free PMC article. Review.
-
Enhancing antioxidant delivery through 3D printing: a pathway to advanced therapeutic strategies.Front Bioeng Biotechnol. 2023 Oct 4;11:1256361. doi: 10.3389/fbioe.2023.1256361. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37860625 Free PMC article. Review.
-
Metallothionein 1: A New Spotlight on Inflammatory Diseases.Front Immunol. 2021 Nov 5;12:739918. doi: 10.3389/fimmu.2021.739918. eCollection 2021. Front Immunol. 2021. PMID: 34804020 Free PMC article. Review.
-
Altered secretory and neuroprotective function of the choroid plexus in progressive multiple sclerosis.Acta Neuropathol Commun. 2020 Mar 19;8(1):35. doi: 10.1186/s40478-020-00903-y. Acta Neuropathol Commun. 2020. PMID: 32192527 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

