Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans
- PMID: 30120234
- PMCID: PMC6098069
- DOI: 10.1038/s41467-018-05770-9
Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans
Abstract
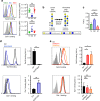
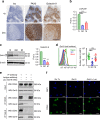
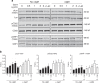
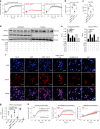
Leukocytes are coated with a layer of heterogeneous carbohydrates (glycans) that modulate immune function, in part by governing specific interactions with glycan-binding proteins (lectins). Although nearly all membrane proteins bear glycans, the identity and function of most of these sugars on leukocytes remain unexplored. Here, we characterize the N-glycan repertoire (N-glycome) of human tonsillar B cells. We observe that naive and memory B cells express an N-glycan repertoire conferring strong binding to the immunoregulatory lectin galectin-9 (Gal-9). Germinal center B cells, by contrast, show sharply diminished binding to Gal-9 due to upregulation of I-branched N-glycans, catalyzed by the β1,6-N-acetylglucosaminyltransferase GCNT2. Functionally, we find that Gal-9 is autologously produced by naive B cells, binds CD45, suppresses calcium signaling via a Lyn-CD22-SHP-1 dependent mechanism, and blunts B cell activation. Thus, our findings suggest Gal-9 intrinsically regulates B cell activation and may differentially modulate BCR signaling at steady state and within germinal centers.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
B Cell Siglecs-News on Signaling and Its Interplay With Ligand Binding.Front Immunol. 2018 Dec 3;9:2820. doi: 10.3389/fimmu.2018.02820. eCollection 2018. Front Immunol. 2018. PMID: 30559744 Free PMC article. Review.
-
ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling.Mol Cell Biol. 2006 Jul;26(13):4970-81. doi: 10.1128/MCB.00308-06. Mol Cell Biol. 2006. PMID: 16782884 Free PMC article.
-
Galectin-9 binds IgM-BCR to regulate B cell signaling.Nat Commun. 2018 Aug 17;9(1):3288. doi: 10.1038/s41467-018-05771-8. Nat Commun. 2018. PMID: 30120235 Free PMC article.
-
Novel binding site for Src homology 2-containing protein-tyrosine phosphatase-1 in CD22 activated by B lymphocyte stimulation with antigen.J Biol Chem. 2008 Jan 18;283(3):1653-1659. doi: 10.1074/jbc.M706584200. Epub 2007 Nov 15. J Biol Chem. 2008. PMID: 18024433
-
CD22 and Siglec-G regulate inhibition of B-cell signaling by sialic acid ligand binding and control B-cell tolerance.Glycobiology. 2014 Sep;24(9):807-17. doi: 10.1093/glycob/cwu066. Epub 2014 Jul 6. Glycobiology. 2014. PMID: 25002414 Review.
Cited by
-
ST8Sia2 polysialyltransferase protects against infection by Trypanosoma cruzi.PLoS Negl Trop Dis. 2024 Sep 25;18(9):e0012454. doi: 10.1371/journal.pntd.0012454. eCollection 2024 Sep. PLoS Negl Trop Dis. 2024. PMID: 39321148 Free PMC article.
-
The role of galectins in immunity and infection.Nat Rev Immunol. 2023 Aug;23(8):479-494. doi: 10.1038/s41577-022-00829-7. Epub 2023 Jan 16. Nat Rev Immunol. 2023. PMID: 36646848 Free PMC article. Review.
-
B Cell Siglecs-News on Signaling and Its Interplay With Ligand Binding.Front Immunol. 2018 Dec 3;9:2820. doi: 10.3389/fimmu.2018.02820. eCollection 2018. Front Immunol. 2018. PMID: 30559744 Free PMC article. Review.
-
Galectin-9 recognizes and exhibits antimicrobial activity toward microbes expressing blood group-like antigens.J Biol Chem. 2022 Apr;298(4):101704. doi: 10.1016/j.jbc.2022.101704. Epub 2022 Feb 9. J Biol Chem. 2022. PMID: 35148986 Free PMC article.
-
Analysis of Galectin-Binding Receptors on B Cells.Methods Mol Biol. 2022;2442:565-580. doi: 10.1007/978-1-0716-2055-7_30. Methods Mol Biol. 2022. PMID: 35320546 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

