Epstein-Barr Virus-Associated Malignancies: Roles of Viral Oncoproteins in Carcinogenesis
- PMID: 30116721
- PMCID: PMC6082928
- DOI: 10.3389/fonc.2018.00265
Epstein-Barr Virus-Associated Malignancies: Roles of Viral Oncoproteins in Carcinogenesis
Abstract
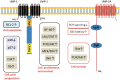
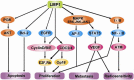
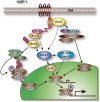
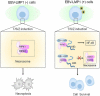
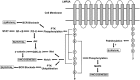
The Epstein-Barr virus (EBV) is the first herpesvirus identified to be associated with human cancers known to infect the majority of the world population. EBV-associated malignancies are associated with a latent form of infection, and several of the EBV-encoded latent proteins are known to mediate cellular transformation. These include six nuclear antigens and three latent membrane proteins (LMPs). In lymphoid and epithelial tumors, viral latent gene expressions have distinct pattern. In both primary and metastatic tumors, the constant expression of latent membrane protein 2A (LMP2A) at the RNA level suggests that this protein is the key player in the EBV-associated tumorigenesis. While LMP2A contributing to the malignant transformation possibly by cooperating with the aberrant host genome. This can be done in part by dysregulating signaling pathways at multiple points, notably in the cell cycle and apoptotic pathways. Recent studies also have confirmed that LMP1 and LMP2 contribute to carcinoma progression and that this may reflect the combined effects of these proteins on activation of multiple signaling pathways. This review article aims to investigate the aforementioned EBV-encoded proteins that reveal established roles in tumor formation, with a greater emphasis on the oncogenic LMPs (LMP1 and LMP2A) and their roles in dysregulating signaling pathways. It also aims to provide a quick look on the six members of the EBV nuclear antigens and their roles in dysregulating apoptosis.
Keywords: B-cells lymphoma; Burkitt’s lymphoma; Hodgkin’s lymphoma; nasopharyngeal carcinoma; non-Hodgkin’s lymphoma; oncogenes; oncoproteins.
Figures

Similar articles
-
The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer.Cell Mol Biol Lett. 2009;14(2):222-47. doi: 10.2478/s11658-008-0045-2. Epub 2008 Dec 13. Cell Mol Biol Lett. 2009. PMID: 19082921 Free PMC article. Review.
-
Regulation of DNA Damage Signaling and Cell Death Responses by Epstein-Barr Virus Latent Membrane Protein 1 (LMP1) and LMP2A in Nasopharyngeal Carcinoma Cells.J Virol. 2015 Aug;89(15):7612-24. doi: 10.1128/JVI.00958-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972552 Free PMC article.
-
Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells.J Exp Med. 1993 Feb 1;177(2):339-49. doi: 10.1084/jem.177.2.339. J Exp Med. 1993. PMID: 8381153 Free PMC article.
-
[Mechanisms of Epstein-Barr Virus-Mediated Oncogenesis].Gan To Kagaku Ryoho. 2015 Oct;42(10):1133-6. Gan To Kagaku Ryoho. 2015. PMID: 26489540 Review. Japanese.
-
[Molecular mechanisms of Epstein-Barr virus-mediated carcinogeneis].Uirusu. 2014;64(1):49-56. doi: 10.2222/jsv.64.49. Uirusu. 2014. PMID: 25765980 Review. Japanese.
Cited by
-
Plasmablastic Lymphoma. A State-of-the-Art Review: Part 1-Epidemiology, Pathogenesis, Clinicopathologic Characteristics, Differential Diagnosis, Prognostic Factors, and Special Populations.Mediterr J Hematol Infect Dis. 2024 Jan 1;16(1):e2024007. doi: 10.4084/MJHID.2024.007. eCollection 2024. Mediterr J Hematol Infect Dis. 2024. PMID: 38223486 Free PMC article. Review.
-
Analysis of Epstein-Barr Virus Infection in Oral Potentially Malignant Disorders and Oral Cancer: A Cross-Sectional Study.J Int Soc Prev Community Dent. 2023 Jun 29;13(3):221-228. doi: 10.4103/jispcd.JISPCD_235_22. eCollection 2023 May-Jun. J Int Soc Prev Community Dent. 2023. PMID: 37564166 Free PMC article.
-
Epstein-Barr virus in gastric cancer and association with 30 bp del-latent membrane protein 1 polymorphism.Rev Assoc Med Bras (1992). 2023 May 19;69(5):e20221571. doi: 10.1590/1806-9282.20221571. eCollection 2023. Rev Assoc Med Bras (1992). 2023. PMID: 37222327 Free PMC article.
-
Contribution of Epstein-Barr Virus Lytic Proteins to Cancer Hallmarks and Implications from Other Oncoviruses.Cancers (Basel). 2023 Apr 2;15(7):2120. doi: 10.3390/cancers15072120. Cancers (Basel). 2023. PMID: 37046781 Free PMC article. Review.
-
Lifestyle, Epstein-Barr virus infection, and other factors could impede nasopharyngeal cancer survivorship: a five-year cross-sectional study in North Eastern India.Virusdisease. 2022 Dec;33(4):371-382. doi: 10.1007/s13337-022-00789-5. Epub 2022 Nov 8. Virusdisease. 2022. PMID: 36447816 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

