Prior exercise training improves cold tolerance independent of indices associated with non-shivering thermogenesis
- PMID: 30109697
- PMCID: PMC6138291
- DOI: 10.1113/JP276228
Prior exercise training improves cold tolerance independent of indices associated with non-shivering thermogenesis
Abstract
Key points: Mammals defend against cold-induced reductions in body temperature through both shivering and non-shivering thermogenesis. The activation of non-shivering thermogenesis is primarily driven by uncoupling protein-1 in brown adipose tissue and to a lesser degree by the browning of white adipose tissue. Endurance exercise has also been shown to increase markers of white adipose tissue browning. This study aimed to determine whether prior exercise training would alter the response to a cold challenge and if this would be associated with differences in indices of non-shivering thermogenesis. It is shown that exercise training protects against cold-induced weight loss by increasing food intake. Exercise-trained mice were better able to maintain their core temperature, independent of differences in markers of non-shivering thermogenesis.
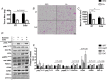
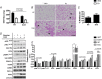
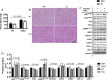
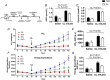
Abstract: Shivering is one of the first defences against cold, and as skeletal muscle fatigues there is an increased reliance on non-shivering thermogenesis. Brown and beige adipose tissues are the primary thermogenic tissues regulating this process. Exercise has also been shown to increase the thermogenic capacity of subcutaneous white adipose tissue. Whether exercise has an effect on the adaptations to cold stress within adipose tissue and skeletal muscle remains to be shown. Male C57BL/6 mice were either subjected to voluntary wheel running or remained sedentary for 12 days. Exercise led to decreased body weight and increased glucose tolerance. Mice were then divided into groups kept at 25°C room temperature or a cold challenge of 4°C for 48 h. Exercised mice were protected against cold-induced reductions in weight and in parallel with increased food intake. Providing exercised mice with the same amount of food as sedentary mice eliminated the protection against cold-induced weight loss. Cold exposure led to greater reductions in rectal temperature in sedentary compared to exercised mice. This protective effect was not explained by differences in the browning of white adipose tissue or brown adipose tissue mass. Similarly, the ability of the β3 -adrenergic agonist CL 316,243 to increase energy expenditure was attenuated in previously exercised mice, suggesting that the activation of uncoupling protein-1 in brown and/or beige adipocytes is not the source of protective effects. We speculate that the protection against cold-induced reductions in rectal temperature could potentially be linked to exercise-induced alterations in skeletal muscle.
Keywords: cold exposure; exercise; thermogenesis.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures




Similar articles
-
Cold acclimation and pioglitazone combined increase thermogenic capacity of brown and white adipose tissues but this does not translate into higher energy expenditure in mice.Am J Physiol Endocrinol Metab. 2023 Apr 1;324(4):E358-E373. doi: 10.1152/ajpendo.00217.2022. Epub 2023 Mar 1. Am J Physiol Endocrinol Metab. 2023. PMID: 36856189
-
Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice.J Physiol. 2019 Sep;597(17):4581-4600. doi: 10.1113/JP278221. Epub 2019 Jul 11. J Physiol. 2019. PMID: 31297830
-
Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue.J Physiol. 2017 Mar 15;595(6):2099-2113. doi: 10.1113/JP273395. Epub 2017 Feb 5. J Physiol. 2017. PMID: 28025824 Free PMC article.
-
Many Ways to Rome: Exercise, Cold Exposure and Diet-Do They All Affect BAT Activation and WAT Browning in the Same Manner?Int J Mol Sci. 2022 Apr 26;23(9):4759. doi: 10.3390/ijms23094759. Int J Mol Sci. 2022. PMID: 35563150 Free PMC article. Review.
-
Thermogenic mechanisms in cold-acclimated animals.Braz J Med Biol Res. 1988;21(2):171-6. Braz J Med Biol Res. 1988. PMID: 3060204 Review.
Cited by
-
Exercise-Induced Adaptations to Adipose Tissue Thermogenesis.Front Endocrinol (Lausanne). 2020 Apr 29;11:270. doi: 10.3389/fendo.2020.00270. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32411099 Free PMC article. Review.
-
Epinephrine responsiveness is reduced in livers from trained mice.Physiol Rep. 2020 Feb;8(3):e14370. doi: 10.14814/phy2.14370. Physiol Rep. 2020. PMID: 32061187 Free PMC article.
-
Exercise-induced intertissue communication: adipose tissue and the heart.Curr Opin Physiol. 2023 Feb;31:100626. doi: 10.1016/j.cophys.2022.100626. Epub 2022 Dec 17. Curr Opin Physiol. 2023. PMID: 36588657 Free PMC article.
-
Chronic exercise mitigates disease mechanisms and improves muscle function in myotonic dystrophy type 1 mice.J Physiol. 2019 Mar;597(5):1361-1381. doi: 10.1113/JP277123. Epub 2019 Jan 30. J Physiol. 2019. PMID: 30628727 Free PMC article.
-
Exercise-induced regulation of adipose tissue.Curr Opin Genet Dev. 2023 Aug;81:102058. doi: 10.1016/j.gde.2023.102058. Epub 2023 Jun 7. Curr Opin Genet Dev. 2023. PMID: 37295241 Free PMC article. Review.
References
-
- Arnold J, LeBlanc J, Cote J, Lalonde J & Richard D (1986). Exercise suppression of thermoregulatory thermogenesis in warm‐ and cold‐acclimated rats. Can J Physiol Pharmacol 64, 922–926. - PubMed
-
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M & Heeren J (2011). Brown adipose tissue activity controls triglyceride clearance. Nat Med 17, 200–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

