Criterion for trismus in head and neck cancer patients: a verification study
- PMID: 30109487
- PMCID: PMC6373229
- DOI: 10.1007/s00520-018-4402-z
Criterion for trismus in head and neck cancer patients: a verification study
Abstract
Purpose: Several cut-off points for trismus in head and neck cancer patients have been used. A mouth opening of 35 mm or less is most frequently used as cut-off point. Due to the variation in cut-off points, prevalence, risk factors and treatment outcomes of trismus cannot be studied in a uniform manner. To provide uniformity, we aimed to verify the cut-off point of 35 mm or less. Additionally, we aimed to determine associated covariates with reported difficulties when opening the mouth.
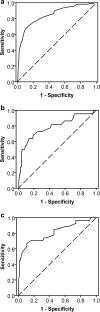
Methods: In a cross-sectional design, we measured the mouth opening in 671 head and neck cancer patients at the Department of Oral and Maxillofacial Surgery, at the University Medical Center Groningen. The cut-off point was determined using the receiver operating characteristic curve and Youden index, with reported difficulties when opening the mouth as criterion for trismus. Cut-off points for significant covariates were also determined.
Results: The Youden index was highest at 35 mm, with a sensitivity of 0.71 and a specificity of 0.86. Of the covariates analysed, type of treatment modality was significantly associated with reported difficulties when opening the mouth. The highest Youden index for patients treated with surgery alone was 37 mm and for patients treated with radiotherapy alone 33 mm.
Conclusions: The cut-off point of 35 mm or less for trismus was confirmed in a head and neck cancer population and is recommended to be used in future studies. Patients receiving different treatment modalities experience difficulty when opening the mouth differently.
Keywords: Head and neck neoplasms; Mouth neoplasms; Quality of life; Range of motion, articular; Surgery, oral; Trismus.
Conflict of interest statement
Research involving human participants and/or animals
Retrospective study: for this type of study formal consent is not required.
The Medical Ethical Committee of the UMCG concluded that our research was not subject to the Medical Research (Human Subject) Act (METc number 2016.692).
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
None declared.
Data
Corresponding author has full control of all primary data.
Corresponding author allows the journal to review the data if requested.
Figures

Similar articles
-
Criteria for trismus in head and neck oncology.Int J Oral Maxillofac Surg. 2006 Apr;35(4):337-42. doi: 10.1016/j.ijom.2005.08.001. Epub 2005 Nov 8. Int J Oral Maxillofac Surg. 2006. PMID: 16280237
-
Temporal patterns of patient-reported trismus and associated mouth-opening distances in radiotherapy for head and neck cancer: A prospective cohort study.Clin Otolaryngol. 2018 Feb;43(1):22-30. doi: 10.1111/coa.12896. Epub 2017 May 29. Clin Otolaryngol. 2018. PMID: 28463432 Free PMC article.
-
Mouth opening and trismus in patients undergoing curative treatment for head and neck cancer.Int J Oral Maxillofac Surg. 2015 Mar;44(3):292-6. doi: 10.1016/j.ijom.2014.12.009. Epub 2015 Jan 7. Int J Oral Maxillofac Surg. 2015. PMID: 25577664
-
Trismus in patients with head and neck cancer: etiopathogenesis, diagnosis and management.Clin Otolaryngol. 2015 Dec;40(6):516-26. doi: 10.1111/coa.12488. Clin Otolaryngol. 2015. PMID: 26098612 Review.
-
Trismus in head and neck oncology: a systematic review.Oral Oncol. 2004 Oct;40(9):879-89. doi: 10.1016/j.oraloncology.2004.04.003. Oral Oncol. 2004. PMID: 15380165 Review.
Cited by
-
Trismus Occurrence and Link With Radiotherapy Doses in Head and Neck Cancer Patients Treated With Chemoradiotherapy.Integr Cancer Ther. 2023 Jan-Dec;22:15347354221147283. doi: 10.1177/15347354221147283. Integr Cancer Ther. 2023. PMID: 36625502 Free PMC article.
-
Oral and dental late effects in long-term survivors of childhood embryonal brain tumors.Support Care Cancer. 2022 Dec;30(12):10233-10241. doi: 10.1007/s00520-022-07405-8. Epub 2022 Oct 29. Support Care Cancer. 2022. PMID: 36307656 Free PMC article.
-
Long-term swallow outcomes and factors affecting swallowing dysfunction and quality of life among oral cancer patients: a prospective observational study.Eur Arch Otorhinolaryngol. 2023 Nov;280(11):5091-5100. doi: 10.1007/s00405-023-08155-x. Epub 2023 Aug 7. Eur Arch Otorhinolaryngol. 2023. PMID: 37548702
-
Prognostic factors associated with a restricted mouth opening (trismus) in patients with head and neck cancer: Systematic review.Head Neck. 2020 Sep;42(9):2696-2721. doi: 10.1002/hed.26327. Epub 2020 Jun 18. Head Neck. 2020. PMID: 32558025 Free PMC article. Review.
-
Effectiveness of mHealth intervention for trismus exercise in patients with head and neck cancer undergoing proton and heavy ion therapy: a randomized control trial.Support Care Cancer. 2024 Jun 29;32(7):470. doi: 10.1007/s00520-024-08679-w. Support Care Cancer. 2024. PMID: 38951291 Clinical Trial.
References
-
- Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT, Trismus Section, Oral Care Study Group, Multinational Association for Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010;18:1033–1038. doi: 10.1007/s00520-010-0847-4. - DOI - PubMed
-
- Melchers LJ, Van Weert E, Beurskens CHG, Reintsema H, Slagter AP, Roodenburg JLN, Dijkstra PU. Exercise adherence in patients with trismus due to head and neck oncology: a qualitative study into the use of the Therabite®. Int J Oral Maxillofac Surg. 2009;38:947–954. doi: 10.1016/j.ijom.2009.04.003. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

