Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8+ T Cells
- PMID: 30108585
- PMCID: PMC6079237
- DOI: 10.3389/fimmu.2018.01751
Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8+ T Cells
Abstract
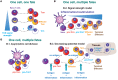
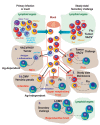
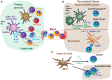
Tissue-resident memory CD8+ T (Trm) cells define a distinct non-recirculating subset. Trm cells constitute a first line of defense against local infections in barrier tissues, but they are also found in non-barrier tissues and play a role in antitumor immunity. Their differentiation in tissues and their phenotypical, transcriptional, and functional characteristics are the object of active research. Herein, we will discuss the potential existence of committed CD8+ Trm precursors and the genealogy of memory CD8+ T cell subsets. In addition to the priming of naive T cells, there is some plasticity of antigen-experienced effector and memory T cell subsets to generate Trm precursors. Local inflammation, antigen presentation, and cytokines drive Trm differentiation. It is of prime interest how specific dendritic cell subsets modulate priming and differentiation of Trm cells, as well as their reactivation within tissues. Research on how we can manipulate generation of memory T cells subsets is key for improved vaccination strategies.
Keywords: circulating memory; dendritic cells; differentiation; infection; memory CD8+ T cell; plasticity; priming; tissue-resident memory.
Figures
Similar articles
-
Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells.Nat Commun. 2017 Jul 17;8:16073. doi: 10.1038/ncomms16073. Nat Commun. 2017. PMID: 28714465 Free PMC article.
-
Armed and Ready: Transcriptional Regulation of Tissue-Resident Memory CD8 T Cells.Front Immunol. 2018 Jul 30;9:1770. doi: 10.3389/fimmu.2018.01770. eCollection 2018. Front Immunol. 2018. PMID: 30131803 Free PMC article. Review.
-
Cross-Presentation of Skin-Targeted Recombinant Adeno-associated Virus 2/1 Transgene Induces Potent Resident Memory CD8+ T Cell Responses.J Virol. 2019 Feb 19;93(5):e01334-18. doi: 10.1128/JVI.01334-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541847 Free PMC article.
-
A Context-Dependent Role for IL-21 in Modulating the Differentiation, Distribution, and Abundance of Effector and Memory CD8 T Cell Subsets.J Immunol. 2016 Mar 1;196(5):2153-66. doi: 10.4049/jimmunol.1401236. Epub 2016 Jan 29. J Immunol. 2016. PMID: 26826252 Free PMC article.
-
Stem cell-like plasticity of naïve and distinct memory CD8+ T cell subsets.Semin Immunol. 2009 Apr;21(2):62-8. doi: 10.1016/j.smim.2009.02.004. Epub 2009 Mar 9. Semin Immunol. 2009. PMID: 19269852 Review.
Cited by
-
Predicted limited redistribution of T cells to secondary lymphoid tissue correlates with increased risk of haematological malignancies in asplenic patients.Sci Rep. 2021 Aug 12;11(1):16394. doi: 10.1038/s41598-021-95225-x. Sci Rep. 2021. PMID: 34385480 Free PMC article.
-
Interactions of Tissue-Resident T Cells.Methods Mol Biol. 2023;2654:437-452. doi: 10.1007/978-1-0716-3135-5_28. Methods Mol Biol. 2023. PMID: 37106199
-
Location versus ID: what matters to lung-resident memory T cells?Front Immunol. 2024 Feb 5;15:1355910. doi: 10.3389/fimmu.2024.1355910. eCollection 2024. Front Immunol. 2024. PMID: 38375476 Free PMC article.
-
Tissue-resident memory T cells in gastrointestinal cancer immunology and immunotherapy: ready for prime time?J Immunother Cancer. 2022 Apr;10(4):e003472. doi: 10.1136/jitc-2021-003472. J Immunother Cancer. 2022. PMID: 35470231 Free PMC article. Review.
-
Tissue-Resident Macrophages Limit Pulmonary CD8 Resident Memory T Cell Establishment.Front Immunol. 2019 Oct 10;10:2332. doi: 10.3389/fimmu.2019.02332. eCollection 2019. Front Immunol. 2019. PMID: 31681267 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

