Serum- and glucocorticoid-inducible kinase 3 is a potential oncogene in nasopharyngeal carcinoma
- PMID: 30108027
- PMCID: PMC9443024
- DOI: 10.1016/j.bjorl.2018.05.012
Serum- and glucocorticoid-inducible kinase 3 is a potential oncogene in nasopharyngeal carcinoma
Abstract
Introduction: Serum- and glucocorticoid-inducible kinase 3, a serine/threonine kinase that functions downstream of the PI3K signaling pathway, plays a critical role in neoplastic processes. It is expressed by various tumors and contributes to carcinogenesis.
Objective: The objective was to investigate serum- and glucocorticoid-inducible kinase 3 expression in nasopharyngeal carcinoma, to study the anti-tumor effects of serum- and glucocorticoid-inducible kinase 3 shRNA by inhibiting its expression in nasopharyngeal carcinoma cells and to discuss the potential implications of our findings.
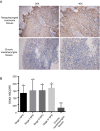
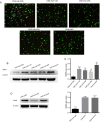
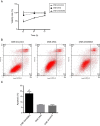
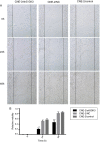
Methods: Serum- and glucocorticoid-inducible kinase 3 protein expression in nasopharyngeal carcinoma cell lines (CNE-1, CNE-2, HNE-1, HONE-1, and SUNE-1) and the human immortalized nasopharyngeal epithelium cell line NP69 were assayed by western blotting. Serum- and glucocorticoid-inducible kinase 3 expression in 42 paraffin-embedded nasopharyngeal carcinoma tissues were performed by immunohistochemistry. MTT assay, flow cytometry, and scratch tests were performed after CNE-2 cells were transfected with the best serum- and glucocorticoid-inducible kinase 3 shRNA plasmid selected by western blotting using lipofectamine to study its effect on cell proliferation, apoptosis, and migration.
Results: Serum- and glucocorticoid-inducible kinase 3 was overexpressed in human nasopharyngeal carcinoma tissues and cells. Serum- and glucocorticoid-inducible kinase 3 expression decreased markedly after CNE-2 cells were transfected with the serum- and glucocorticoid-inducible kinase 3 shRNA, leading to strong inhibition of cell proliferation and migration. In addition, the apoptosis rate increased in CNE-2 cells after serum- and glucocorticoid-inducible kinase 3 knockdown.
Conclusion: Serum- and glucocorticoid-inducible kinase 3 expression was more frequently observed as the nasopharyngeal epithelium progresses from normal tissue to carcinoma. This suggests that serum- and glucocorticoid-inducible kinase 3 contributes to the multistep process of NPC carcinogenesis. Serum- and glucocorticoid-inducible kinase 3 represents a target for nasopharyngeal carcinoma therapy, and a basis exists for the further investigation of this adjuvant treatment modality for nasopharyngeal carcinoma.
Introdução: A quinase 3 sérica induzida por glicocorticoide, uma serina/treonina quinase que funciona downstream da via de sinalização PI3K, desempenha um papel crítico nos processos neoplásicos. É expressa por vários tumores e contribui para a carcinogênese.
Objetivo: O objetivo foi investigar a expressão de quinase 3 sérica induzida por glicocorticoide no carcinoma nasofaríngeo, estudar os efeitos antitumorais do shRNA da quinase 3 sérica induzida por glicocorticoide, inibindo sua expressão em células de carcinoma nasofaríngeo e discutir as implicações potenciais de nossos achados.
Método: A expressão de proteína quinase 3 sérica induzida por glicocorticoide em linhagens de células de carcinoma nasofaríngeo (CNE-1, CNE-2, HNE-1, HONE-1 e SUNE-1) e a linhagem de células humanas imortalizadas do epitélio nasofaríngeo NP69 foram avaliadas por Western blot. A expressão da quinase 3 sérica induzida por glicocorticoide em 42 tecidos de CNF embebidos em parafina foi realizada por imunohistoquímica. Testes com MTT, citometria de fluxo e testes de raspagem foram realizados após as células CNE-2 terem sido transfectadas com o melhor plasmídeo shRNA da quinase 3 sérica induzida por glicocorticoide selecionado por Western blot, utilizando lipofectamina para estudar seu efeito na proliferação, apoptose e migração celular.
Resultados: Foi observada uma sobre-expressão da quinase 3 sérica induzida por glicocorticoide em tecidos e células de carcinoma nasofaríngeo humanas. A expressão de quinase 3 sérica induzida por glicocorticoide diminuiu acentuadamente após as células CNE-2 terem sido transfectadas com o shRNA da quinase 3 sérica induzida por glicocorticoide, conduzindo à forte inibição de proliferação e migração celular. Além disso, a taxa de apoptose aumentou nas células CNE-2 após o knockdown da quinase 3 sérica induzida por glicocorticoide.
Conclusão: A expressão de quinase 3 sérica induzida por glicocorticoide foi observada com maior frequência à medida que o epitélio nasofaríngeo progride de tecido normal para carcinoma. Isto sugere que a quinase 3 sérica induzida por glicocorticoide contribui para o processo multi-etapas da carcinogênese do carcinoma nasofaríngeo. A quinase 3 sérica induzida por glicocorticoide representa um alvo para a terapia do carcinoma nasofaríngeoe há uma base para a investigação adicional desta modalidade de tratamento adjuvante para o carcinoma nasofaríngeo.
Keywords: Apoptose; Apoptosis; Carcinoma nasofaríngeo; Migration; Migração; Nasopharyngeal carcinoma; Proliferation; Proliferação; Quinase 3 sérica e induzida por glicocorticoide; Serum- and glucocorticoid-inducible kinase 3; shRNA.
Copyright © 2018 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Published by Elsevier Editora Ltda. All rights reserved.
Figures

Similar articles
-
miR-331-3p Inhibits Proliferation and Promotes Apoptosis of Nasopharyngeal Carcinoma Cells by Targeting elf4B-PI3K-AKT Pathway.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033819892251. doi: 10.1177/1533033819892251. Technol Cancer Res Treat. 2020. Retraction in: Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221109185. doi: 10.1177/15330338221109185. PMID: 31984860 Free PMC article. Retracted.
-
MicroRNA-185 inhibits cell proliferation while promoting apoptosis and autophagy through negative regulation of TGF-β1/mTOR axis and HOXC6 in nasopharyngeal carcinoma.Cancer Biomark. 2018;23(1):107-123. doi: 10.3233/CBM-181459. Cancer Biomark. 2018. Retraction in: Cancer Biomark. 2022;34(4):695. doi: 10.3233/CBM-220951. PMID: 29991129 Retracted.
-
The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis.Molecules. 2021 Mar 14;26(6):1604. doi: 10.3390/molecules26061604. Molecules. 2021. PMID: 33799348 Free PMC article.
-
ShRNA-mediated silencing of the ubiquitin-specific protease 22 gene restrained cell progression and affected the Akt pathway in nasopharyngeal carcinoma.Cancer Biol Ther. 2015;16(1):88-96. doi: 10.4161/15384047.2014.987029. Cancer Biol Ther. 2015. PMID: 25482932 Free PMC article.
-
MiR-101 promotes nasopharyngeal carcinoma cell apoptosis through inhibiting Ras/Raf/MEK/ERK signaling pathway.Eur Rev Med Pharmacol Sci. 2018 Jan;22(1):150-157. doi: 10.26355/eurrev_201801_14112. Eur Rev Med Pharmacol Sci. 2018. Retraction in: Eur Rev Med Pharmacol Sci. 2020 Aug;24(16):8240. doi: 10.26355/eurrev_202008_22580. PMID: 29364482 Retracted.
Cited by
-
Major Stressful Life Events and the Risk of Pancreatic, Head and Neck Cancers: A Case-Control Study.Cancers (Basel). 2024 Jan 20;16(2):451. doi: 10.3390/cancers16020451. Cancers (Basel). 2024. PMID: 38275892 Free PMC article.
-
Cell Proliferation and Apoptosis-Related Genes Affect the Development of Human Nasopharyngeal Carcinoma Through PI3K/AKT Signaling Pathway.Mol Biotechnol. 2021 Nov;63(11):1081-1091. doi: 10.1007/s12033-021-00357-0. Epub 2021 Jul 8. Mol Biotechnol. 2021. PMID: 34236626
-
Cancer and Stress: Does It Make a Difference to the Patient When These Two Challenges Collide?Cancers (Basel). 2021 Jan 6;13(2):163. doi: 10.3390/cancers13020163. Cancers (Basel). 2021. PMID: 33418900 Free PMC article. Review.
-
The Role of hsa-miR-21 and Its Target Genes Involved in Nasopharyngeal Carcinoma.Asian Pac J Cancer Prev. 2021 Dec 1;22(12):4075-4083. doi: 10.31557/APJCP.2021.22.12.4075. Asian Pac J Cancer Prev. 2021. PMID: 34967592 Free PMC article.
References
-
- Chua M.L., Wee J.T., Hui E.P., Chan A.T. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. - PubMed
-
- Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Fong K.W., Chua E.J., Chua E.T., Khoo-Tan H.S., Lee K.M., Lee K.S., et al. Patient profile and survival in 270 computer tomography-staged patients with nasopharyngeal cancer treated at the Singapore General Hospital. Ann Acad Med Singapore. 1996;25:341–346. - PubMed
-
- Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., et al. Cancer statistics in China. CA Cancer J Clin. 2016;66:115–132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

