Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System
- PMID: 30104956
- PMCID: PMC6077210
- DOI: 10.3389/fnmol.2018.00258
Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System
Abstract
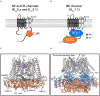
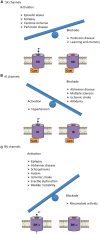
Within the potassium ion channel family, calcium activated potassium (KCa) channels are unique in their ability to couple intracellular Ca2+ signals to membrane potential variations. KCa channels are diversely distributed throughout the central nervous system and play fundamental roles ranging from regulating neuronal excitability to controlling neurotransmitter release. The physiological versatility of KCa channels is enhanced by alternative splicing and co-assembly with auxiliary subunits, leading to fundamental differences in distribution, subunit composition and pharmacological profiles. Thus, understanding specific KCa channels' mechanisms in neuronal function is challenging. Based on their single channel conductance, KCa channels are divided into three subtypes: small (SK, 4-14 pS), intermediate (IK, 32-39 pS) and big potassium (BK, 200-300 pS) channels. This review describes the biophysical characteristics of these KCa channels, as well as their physiological roles and pathological implications. In addition, we also discuss the current pharmacological strategies and challenges to target KCa channels for the treatment of various neurological and psychiatric disorders.
Keywords: BK channels; IK channels; SK channels; drug discovery; modulators; nervous system; neurological disease.
Figures
Similar articles
-
KCa-Related Neurological Disorders: Phenotypic Spectrum and Therapeutic Indications.Curr Neuropharmacol. 2023;21(7):1504-1518. doi: 10.2174/1570159X21666221208091805. Curr Neuropharmacol. 2023. PMID: 36503451 Free PMC article. Review.
-
Calcium-Activated K+ Channels (KCa) and Therapeutic Implications.Handb Exp Pharmacol. 2021;267:379-416. doi: 10.1007/164_2021_459. Handb Exp Pharmacol. 2021. PMID: 33945030
-
Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications.Int J Mol Sci. 2024 Mar 4;25(5):2965. doi: 10.3390/ijms25052965. Int J Mol Sci. 2024. PMID: 38474212 Free PMC article. Review.
-
KCa 2.2 (KCNN2): A physiologically and therapeutically important potassium channel.J Neurosci Res. 2023 Nov;101(11):1699-1710. doi: 10.1002/jnr.25233. Epub 2023 Jul 19. J Neurosci Res. 2023. PMID: 37466411 Free PMC article. Review.
-
Targeting the Small- and Intermediate-Conductance Ca-Activated Potassium Channels: The Drug-Binding Pocket at the Channel/Calmodulin Interface.Neurosignals. 2014;22(2):65-78. doi: 10.1159/000367896. Epub 2014 Oct 8. Neurosignals. 2014. PMID: 25300231 Free PMC article. Review.
Cited by
-
Phylogenetic and developmental analyses indicate complex functions of calcium-activated potassium channels in zebrafish embryonic development.Dev Dyn. 2021 Oct;250(10):1477-1493. doi: 10.1002/dvdy.329. Epub 2021 Mar 24. Dev Dyn. 2021. PMID: 33728688 Free PMC article.
-
The role of SK3 in progesterone-induced inhibition of human fallopian tubal contraction.Reprod Biol Endocrinol. 2022 Apr 29;20(1):73. doi: 10.1186/s12958-022-00932-3. Reprod Biol Endocrinol. 2022. PMID: 35488306 Free PMC article.
-
Serotonin receptor expression in hippocampus and temporal cortex of temporal lobe epilepsy patients by postictal generalized electroencephalographic suppression duration.Epilepsia. 2022 Nov;63(11):2925-2936. doi: 10.1111/epi.17400. Epub 2022 Sep 5. Epilepsia. 2022. PMID: 36053862 Free PMC article.
-
Protein kinase CK2 and ion channels (Review).Biomed Rep. 2020 Dec;13(6):55. doi: 10.3892/br.2020.1362. Epub 2020 Sep 30. Biomed Rep. 2020. PMID: 33082952 Free PMC article. Review.
-
The Concise Guide to PHARMACOLOGY 2023/24: Ion channels.Br J Pharmacol. 2023 Oct;180 Suppl 2(Suppl 2):S145-S222. doi: 10.1111/bph.16178. Br J Pharmacol. 2023. PMID: 38123150 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

