Ischemia-modified albumin: Crosstalk between fatty acid and cobalt binding
- PMID: 30103926
- PMCID: PMC6109191
- DOI: 10.1016/j.plefa.2018.07.014
Ischemia-modified albumin: Crosstalk between fatty acid and cobalt binding
Abstract
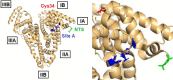
Myocardial ischemia is difficult to diagnose effectively with still few well-defined biochemical markers for identification in advance, or in the absence of myocardial necrosis. "Ischemia-modified albumin" (IMA), a form of albumin displaying reduced cobalt-binding affinity, is significantly elevated in ischemic patients, and the albumin cobalt-binding (ACB) assay can measure its level indirectly. Elucidating the molecular mechanism underlying the identity of IMA and the ACB assay hinges on understanding metal-binding properties of albumin. Albumin binds most metal ions and harbours four primary metal binding sites: site A, site B, the N-terminal site (NTS), and the free thiol at Cys34. Previous efforts to clarify the identity of IMA and the causes for its reduced cobalt-binding capacity were focused on the NTS site, but the degree of N-terminal modification could not be correlated to the presence of ischemia. More recent work suggested that Co2+ ions as used in the ACB assay bind preferentially to site B, then to site A, and finally to the NTS. This insight paved the way for a new consistent molecular basis of the ACB assay: albumin is also the main plasma carrier for free fatty acids (FFAs), and binding of a fatty acid to the high-affinity site FA2 results in conformational changes in albumin which prevent metal binding at site A and partially at site B. Thus, this review advances the hypothesis that high IMA levels in myocardial ischemia and many other conditions originate from high plasma FFA levels hampering the binding of Co2+ to sites A and/or B. This is supported by biophysical studies and the co-association of a range of pathological conditions with positive ACB assays and high plasma FFA levels.
Keywords: Albumin cobalt binding assay; Free fatty acids; Human serum albumin; Molecular diagnostics; Myocardial ischemia.
Copyright © 2018. Published by Elsevier Ltd.
Figures
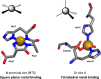
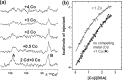
 ) demonstrate that addition of Zn2+ decreases albumin's affinity and capacity for Co2+-binding, while addition of Cu2+ has no significant effect.
) demonstrate that addition of Zn2+ decreases albumin's affinity and capacity for Co2+-binding, while addition of Cu2+ has no significant effect.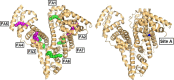
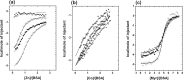
 ) or Co2+ (●) affects the energetics of fatty acid binding relative to the metal-free experiment (○), likely due to the need to remove the metal before the FFA can bind. Notably, again the effect for Zn2+ is larger than that for Co2+.
) or Co2+ (●) affects the energetics of fatty acid binding relative to the metal-free experiment (○), likely due to the need to remove the metal before the FFA can bind. Notably, again the effect for Zn2+ is larger than that for Co2+.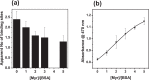
Similar articles
-
Allosteric inhibition of cobalt binding to albumin by fatty acids: implications for the detection of myocardial ischemia.J Med Chem. 2012 May 10;55(9):4425-30. doi: 10.1021/jm3003137. Epub 2012 May 2. J Med Chem. 2012. PMID: 22519414
-
Albumin cobalt binding (ACB) test: its role as a novel marker of acute coronary syndrome.Acta Med Indones. 2006 Apr-Jun;38(2):92-6. Acta Med Indones. 2006. PMID: 16799211 Review.
-
Analytical performance of the Albumin Cobalt Binding (ACB) test on the Cobas MIRA Plus analyzer.Clin Chem Lab Med. 2004 Apr;42(4):455-61. doi: 10.1515/CCLM.2004.079. Clin Chem Lab Med. 2004. PMID: 15147158
-
Evidence that the principal CoII-binding site in human serum albumin is not at the N-terminus: implication on the albumin cobalt binding test for detecting myocardial ischemia.Biochemistry. 2007 Feb 27;46(8):2267-74. doi: 10.1021/bi061783p. Epub 2007 Feb 3. Biochemistry. 2007. PMID: 17274600
-
Allosteric modulation of zinc speciation by fatty acids.Biochim Biophys Acta. 2013 Dec;1830(12):5456-64. doi: 10.1016/j.bbagen.2013.05.028. Epub 2013 May 29. Biochim Biophys Acta. 2013. PMID: 23726993 Review.
Cited by
-
Markers of Oxidative Stress in Patients with Acne: A Literature Review.Life (Basel). 2023 Jun 23;13(7):1433. doi: 10.3390/life13071433. Life (Basel). 2023. PMID: 37511808 Free PMC article. Review.
-
Intestinal fatty acid-binding protein as a biomarker for the diagnosis of strangulated intestinal obstruction: A meta-analysis.Open Med (Wars). 2021 Feb 2;16(1):264-273. doi: 10.1515/med-2021-0214. eCollection 2021. Open Med (Wars). 2021. PMID: 33623822 Free PMC article.
-
Increase in Ischemia-Modified Albumin and Pregnancy-Associated Plasma Protein-A in COVID-19 Patients.J Clin Med. 2021 Nov 23;10(23):5474. doi: 10.3390/jcm10235474. J Clin Med. 2021. PMID: 34884175 Free PMC article.
-
Crosstalk between zinc and free fatty acids in plasma.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Apr;1864(4):532-542. doi: 10.1016/j.bbalip.2018.09.007. Epub 2018 Sep 25. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30266430 Free PMC article. Review.
-
Oxidative Stress Biomarkers in Age-Related Lower Urinary Tract Disorders: A Systematic Review.Int Neurourol J. 2022 Mar;26(1):3-19. doi: 10.5213/inj.2142188.094. Epub 2022 Mar 31. Int Neurourol J. 2022. PMID: 35368181 Free PMC article.
References
-
- Picano E., Palinkas A., Amyot R. Diagnosis of myocardial ischemia in hypertensive patients. J. Hypertens. 2001;19:1177–1183. - PubMed
-
- Apple F.S., Wu A.H., Mair J., Ravkilde J., Panteghini M., Tate J., Pagani F., Christenson R.H., Mockel M., Danne O., Jaffe A.S. I. Committee on standardization of markers of cardiac damage of the, future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin. Chem. 2005;51:810–824. - PubMed
-
- Collinson P.O., Gaze D.C. Biomarkers of cardiovascular damage and dysfunction–an overview. Heart Lung Circ. 2007;16(Suppl 3):S71–S82. - PubMed
-
- Collinson P.O., Gaze D.C. Ischaemia-modified albumin: clinical utility and pitfalls in measurement. J. Clin. Pathol. 2008;61:1025–1028. - PubMed
-
- Gaze D.C. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab. Pharmacokinet. 2009;24:333–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

