Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs
- PMID: 30091119
- PMCID: PMC6082751
- DOI: 10.1186/s40635-018-0194-1
Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs
Abstract
Background: Septic shock is the leading cause of death in intensive care units. The pathophysiological complexity of this syndrome contributes to an absence of specific treatment. Several preclinical studies in murine models of septic shock have shown improvements to organ injury and survival after administration of mesenchymal stem cells (MSCs). To better mimic a clinical approach in humans, we investigated the impact of randomized controlled double-blind administration of clinical-grade umbilical cord-derived MSCs to a relevant pig model of septic shock.
Methods: Septic shock was induced by fecal peritonitis in 12 male domestic pigs. Animals were resuscitated by an experienced intensivist including fluid administration and vasopressors. Four hours after the induction of peritonitis, pigs were randomized to receive intravenous injection of thawed umbilical cord-derived MSCs (UCMSC) (1 × 106 UCMSCs/kg diluted in 75 mL hydroxyethyl starch (HES), (n = 6) or placebo (HES alone, n = 6). Researchers were double-blinded to the treatment administered. Hemodynamic parameters were continuously recorded. Gas exchange, acid-base status, organ function, and plasma cytokine concentrations were assessed at regular intervals until 24 h after the onset of peritonitis when animals were sacrificed under anesthesia.
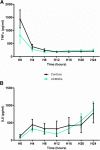
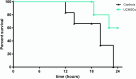
Results: Peritonitis induced profound hypotension, hyperlactatemia, and multiple organ failure. These disorders were significantly attenuated when animals were treated with UCMSCs. In particular, cardiovascular failure was attenuated, as attested by a better mean arterial pressure and reduced lactatemia, despite lower norepinephrine requirements. As such, UCMSCs improved survival in this very severe model (60% survival vs. 0% at 24 h).
Conclusion: UCMSCs administration is beneficial in this pig model of polymicrobial septic shock.
Keywords: Clinical-grade; Mesenchymal stem cells; Septic shock; Umbilical cord.
Conflict of interest statement
Umbilical cords were obtained from new mothers after they had signed an informed consent form in compliance with French national legislation relating to human sample collection, manipulation, and personal data protection. The collection protocol was approved by Nancy Hospital’s ethics committee and the French ministry for research (No. DC-2014-2114).
Experiments were performed in line with the National Institute of Health guidelines on the Use of Laboratory Animals and were approved by the University Animal Care Committee (Comité d’Éthique Lorrain en Matière d’Expérimentation Animale (CELMEA-CE2A-66) authorization no. APAFIS5674-201606141602993).
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effects of a TREM-like transcript 1-derived peptide during hypodynamic septic shock in pigs.Shock. 2013 Feb;39(2):176-82. doi: 10.1097/SHK.0b013e31827bcdfb. Shock. 2013. PMID: 23324887
-
PG2, a botanically derived drug extracted from Astragalus membranaceus, promotes proliferation and immunosuppression of umbilical cord-derived mesenchymal stem cells.J Ethnopharmacol. 2017 Jul 31;207:184-191. doi: 10.1016/j.jep.2017.06.018. Epub 2017 Jun 23. J Ethnopharmacol. 2017. PMID: 28648928
-
Hemodynamic, metabolic, and organ function effects of pure oxygen ventilation during established fecal peritonitis-induced septic shock.Crit Care Med. 2009 Aug;37(8):2465-9. doi: 10.1097/CCM.0b013e3181aee8ad. Crit Care Med. 2009. PMID: 19531939
-
Small-volume hypertonic saline/pentastarch improves ileal mucosal microcirculation in experimental peritonitis.Infect Dis Rep. 2012 Mar 5;4(1):e22. doi: 10.4081/idr.2012.e22. eCollection 2012 Jan 2. Infect Dis Rep. 2012. PMID: 24470929 Free PMC article. Review.
-
Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012.Crit Care Med. 2013 Feb;41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af. Crit Care Med. 2013. PMID: 23353941
Cited by
-
Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs).Int J Mol Sci. 2022 Aug 17;23(16):9274. doi: 10.3390/ijms23169274. Int J Mol Sci. 2022. PMID: 36012544 Free PMC article. Review.
-
Mesenchymal stromal cells for sepsis and septic shock: Lessons for treatment of COVID-19.Stem Cells Transl Med. 2020 Dec;9(12):1488-1494. doi: 10.1002/sctm.20-0239. Epub 2020 Aug 18. Stem Cells Transl Med. 2020. PMID: 32808462 Free PMC article.
-
Scale-Up of Academic Mesenchymal Stromal Cell Production.J Clin Med. 2023 Jun 30;12(13):4414. doi: 10.3390/jcm12134414. J Clin Med. 2023. PMID: 37445448 Free PMC article.
-
Machine learning reveals the rules governing the efficacy of mesenchymal stromal cells in septic preclinical models.Stem Cell Res Ther. 2024 Sep 11;15(1):289. doi: 10.1186/s13287-024-03873-3. Stem Cell Res Ther. 2024. PMID: 39256841 Free PMC article.
-
Combined Mesenchymal Stromal Cell Therapy and Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome. A Randomized Controlled Trial in Sheep.Am J Respir Crit Care Med. 2020 Aug 1;202(3):383-392. doi: 10.1164/rccm.201911-2143OC. Am J Respir Crit Care Med. 2020. PMID: 32293914 Free PMC article.
References
-
- Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:775. doi: 10.1001/jama.2016.0289. - DOI - PMC - PubMed
-
- van Vught LA, Wiewel MA, Hoogendijk AJ, Frencken JF, Scicluna BP, Klein Klouwenberg PMC et al (2017) The host response in sepsis patients developing intensive care unit-acquired secondary infections. Am J Respir Crit Care Med. 10.1164/rccm.201606-1225OC. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

