Direct recognition of hepatocyte-expressed MHC class I alloantigens is required for tolerance induction
- PMID: 30089715
- PMCID: PMC6129134
- DOI: 10.1172/jci.insight.97500
Direct recognition of hepatocyte-expressed MHC class I alloantigens is required for tolerance induction
Abstract
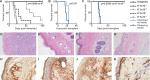
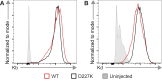
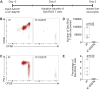
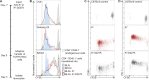
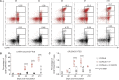
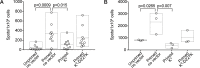
Adeno-associated viral vector-mediated (AAV-mediated) expression of allogeneic major histocompatibility complex class I (MHC class I) in recipient liver induces donor-specific tolerance in mouse skin transplant models in which a class I allele (H-2Kb or H-2Kd) is mismatched between donor and recipient. Tolerance can be induced in mice primed by prior rejection of a donor-strain skin graft, as well as in naive recipients. Allogeneic MHC class I may be recognized by recipient T cells as an intact molecule (direct recognition) or may be processed and presented as an allogeneic peptide in the context of self-MHC (indirect recognition). The relative contributions of direct and indirect allorecognition to tolerance induction in this setting are unknown. Using hepatocyte-specific AAV vectors encoding WT allogeneic MHC class I molecules, or class I molecules containing a point mutation (D227K) that impedes direct recognition of intact allogeneic MHC class I by CD8+ T cells without hampering the presentation of processed peptides derived from allogeneic MHC class I, we show here that tolerance induction depends upon recognition of intact MHC class I. Indirect recognition alone yielded a modest prolongation of subsequent skin graft survival, attributable to the generation of CD4+ Tregs, but it was not sufficient to induce tolerance.
Keywords: Antigen presentation; Gene therapy; Hepatology; Tolerance; Transplantation.
Conflict of interest statement
Figures
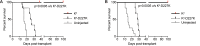



Similar articles
-
Alloantigen gene transfer to hepatocytes promotes tolerance to pancreatic islet graft by inducing CD8+ regulatory T cells.J Hepatol. 2017 Apr;66(4):765-777. doi: 10.1016/j.jhep.2016.11.019. Epub 2016 Nov 30. J Hepatol. 2017. PMID: 27914923
-
Regulatory CD8 T cells that recognize Qa-1 expressed by CD4 T-helper cells inhibit rejection of heart allografts.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6042-6046. doi: 10.1073/pnas.1918950117. Epub 2020 Feb 28. Proc Natl Acad Sci U S A. 2020. PMID: 32111690 Free PMC article.
-
Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation.J Immunol. 2001 Aug 15;167(4):1891-9. doi: 10.4049/jimmunol.167.4.1891. J Immunol. 2001. PMID: 11489968
-
Indirect T cell recognition in allograft rejection.Int Rev Immunol. 1996;13(3):245-55. doi: 10.3109/08830189609061751. Int Rev Immunol. 1996. PMID: 8782745 Review.
-
Indirect allorecognition in solid organ transplantation.Rev Immunogenet. 1999;1(3):297-308. Rev Immunogenet. 1999. PMID: 11256421 Review.
Cited by
-
Mechanisms of Immune Tolerance in Liver Transplantation-Crosstalk Between Alloreactive T Cells and Liver Cells With Therapeutic Prospects.Front Immunol. 2019 Nov 19;10:2667. doi: 10.3389/fimmu.2019.02667. eCollection 2019. Front Immunol. 2019. PMID: 31803188 Free PMC article. Review.
-
Soluble N-Acetylgalactosamine-Modified Antigens Enhance Hepatocyte-Dependent Antigen Cross-Presentation and Result in Antigen-Specific CD8+ T Cell Tolerance Development.Front Immunol. 2021 Mar 3;12:555095. doi: 10.3389/fimmu.2021.555095. eCollection 2021. Front Immunol. 2021. PMID: 33746941 Free PMC article.
-
Dual muscle-liver transduction imposes immune tolerance for muscle transgene engraftment despite preexisting immunity.JCI Insight. 2019 Jun 6;4(11):e127008. doi: 10.1172/jci.insight.127008. eCollection 2019 Jun 6. JCI Insight. 2019. PMID: 31167976 Free PMC article.
-
Maternal hepatic immunology during pregnancy.Front Immunol. 2023 Jun 30;14:1220323. doi: 10.3389/fimmu.2023.1220323. eCollection 2023. Front Immunol. 2023. PMID: 37457700 Free PMC article. Review.
-
Role of the immune system in liver transplantation and its implications for therapeutic interventions.MedComm (2020). 2023 Dec 13;4(6):e444. doi: 10.1002/mco2.444. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38098611 Free PMC article. Review.
References
-
- ANZDATA Registry. 39th Report, Chapter 8: Transplantation. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2017 . http://www.anzdata.org.au/anzdata/AnzdataReport/39thReport/c08_transplan... Accessed July 12, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

