Making Sense of Pharmacology: Inverse Agonism and Functional Selectivity
- PMID: 30085126
- PMCID: PMC6165953
- DOI: 10.1093/ijnp/pyy071
Making Sense of Pharmacology: Inverse Agonism and Functional Selectivity
Abstract
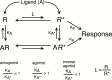
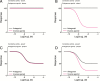
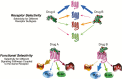
Constitutive receptor activity/inverse agonism and functional selectivity/biased agonism are 2 concepts in contemporary pharmacology that have major implications for the use of drugs in medicine and research as well as for the processes of new drug development. Traditional receptor theory postulated that receptors in a population are quiescent unless activated by a ligand. Within this framework ligands could act as agonists with various degrees of intrinsic efficacy, or as antagonists with zero intrinsic efficacy. We now know that receptors can be active without an activating ligand and thus display "constitutive" activity. As a result, a new class of ligand was discovered that can reduce the constitutive activity of a receptor. These ligands produce the opposite effect of an agonist and are called inverse agonists. The second topic discussed is functional selectivity, also commonly referred to as biased agonism. Traditional receptor theory also posited that intrinsic efficacy is a single drug property independent of the system in which the drug acts. However, we now know that a drug, acting at a single receptor subtype, can have multiple intrinsic efficacies that differ depending on which of the multiple responses coupled to a receptor is measured. Thus, a drug can be simultaneously an agonist, an antagonist, and an inverse agonist acting at the same receptor. This means that drugs have an additional level of selectivity (signaling selectivity or "functional selectivity") beyond the traditional receptor selectivity. Both inverse agonism and functional selectivity need to be considered when drugs are used as medicines or as research tools.
Figures

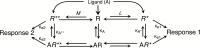
Similar articles
-
Functional selectivity through protean and biased agonism: who steers the ship?Mol Pharmacol. 2007 Dec;72(6):1393-401. doi: 10.1124/mol.107.040352. Epub 2007 Sep 27. Mol Pharmacol. 2007. PMID: 17901198 Review.
-
Inverse agonism at serotonin and cannabinoid receptors.Prog Mol Biol Transl Sci. 2010;91:1-40. doi: 10.1016/S1877-1173(10)91001-6. Prog Mol Biol Transl Sci. 2010. PMID: 20691957 Review.
-
Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors.Pharmacol Ther. 2009 Feb;121(2):160-73. doi: 10.1016/j.pharmthera.2008.10.010. Epub 2008 Dec 6. Pharmacol Ther. 2009. PMID: 19109993 Review.
-
Biased agonism at G protein-coupled receptors: the promise and the challenges--a medicinal chemistry perspective.Med Res Rev. 2014 Nov;34(6):1286-330. doi: 10.1002/med.21318. Epub 2014 May 5. Med Res Rev. 2014. PMID: 24796277 Review.
-
The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-HT7 receptors.Br J Pharmacol. 2015 Aug;172(15):3846-60. doi: 10.1111/bph.13169. Epub 2015 Jun 12. Br J Pharmacol. 2015. PMID: 25884989 Free PMC article.
Cited by
-
Common coupling map advances GPCR-G protein selectivity.Elife. 2022 Mar 18;11:e74107. doi: 10.7554/eLife.74107. Elife. 2022. PMID: 35302494 Free PMC article.
-
Signaling characteristics and functional regulation of delta opioid-kappa opioid receptor (DOP-KOP) heteromers in peripheral sensory neurons.Neuropharmacology. 2019 Jun;151:208-218. doi: 10.1016/j.neuropharm.2019.02.019. Epub 2019 Feb 15. Neuropharmacology. 2019. PMID: 30776373 Free PMC article.
-
Potential of Ligands for Trace Amine-Associated Receptor 1 (TAAR1) in the Management of Substance Use Disorders.CNS Drugs. 2021 Dec;35(12):1239-1248. doi: 10.1007/s40263-021-00871-4. Epub 2021 Nov 12. CNS Drugs. 2021. PMID: 34766253 Free PMC article. Review.
-
Assay-Dependent Inverse Agonism at the A3 Adenosine Receptor: When Neutral Is Not Neutral.ACS Pharmacol Transl Sci. 2023 Aug 9;6(9):1266-1274. doi: 10.1021/acsptsci.3c00071. eCollection 2023 Sep 8. ACS Pharmacol Transl Sci. 2023. PMID: 37705594 Free PMC article.
-
3-Iodothyronamine Affects Thermogenic Substrates' Mobilization in Brown Adipocytes.Biology (Basel). 2020 May 4;9(5):95. doi: 10.3390/biology9050095. Biology (Basel). 2020. PMID: 32375297 Free PMC article.
References
-
- Ahlquist RP.(1948)A study of the adrenotropic receptors. Am J Physiol 153:586–600. - PubMed
-
- Ahn KH, Scott CE, Abrol R, Goddard WA III, Kendall DA(2013)Computationally-predicted CB1 cannabinoid receptor mutants show distinct patterns of salt-bridges that correlate with their level of constitutive activity reflected in G protein coupling levels, thermal stability, and ligand binding. Proteins 81:1304–1317. - PMC - PubMed
-
- Alewijnse AE, Timmerman H, Jacobs EH, Smit MJ, Roovers E, Cotecchia S, Leurs R(2000)The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol Pharmacol 57:890–898. - PubMed
-
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L(2001)Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein homer. Nature 411:962–965. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

