Stress Granules and Processing Bodies in Translational Control
- PMID: 30082464
- PMCID: PMC6496347
- DOI: 10.1101/cshperspect.a032813
Stress Granules and Processing Bodies in Translational Control
Abstract
Stress granules (SGs) and processing bodies (PBs) are non-membrane-enclosed RNA granules that dynamically sequester translationally inactive messenger ribonucleoprotein particles (mRNPs) into compartments that are distinct from the surrounding cytoplasm. mRNP remodeling, silencing, and/or storage involves the dynamic partitioning of closed-loop polyadenylated mRNPs into SGs, or the sequestration of deadenylated, linear mRNPs into PBs. SGs form when stress-activated pathways stall translation initiation but allow elongation and termination to occur normally, resulting in a sudden excess of mRNPs that are spatially condensed into discrete foci by protein:protein, protein:RNA, and RNA:RNA interactions. In contrast, PBs can exist in the absence of stress, when specific factors promote mRNA deadenylation, condensation, and sequestration from the translational machinery. The formation and dissolution of SGs and PBs reflect changes in messenger RNA (mRNA) metabolism and allow cells to modulate the proteome and/or mediate life or death decisions during changing environmental conditions.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

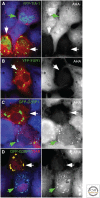
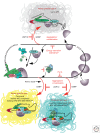
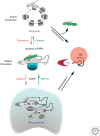
Similar articles
-
Plant stress granules and mRNA processing bodies are distinct from heat stress granules.Plant J. 2008 Nov;56(4):517-30. doi: 10.1111/j.1365-313X.2008.03623.x. Epub 2008 Aug 6. Plant J. 2008. PMID: 18643965
-
Cytoplasmic mRNPs revisited: Singletons and condensates.Bioessays. 2020 Dec;42(12):e2000097. doi: 10.1002/bies.202000097. Epub 2020 Nov 4. Bioessays. 2020. PMID: 33145808
-
Stress granules and processing bodies are dynamically linked sites of mRNP remodeling.J Cell Biol. 2005 Jun 20;169(6):871-84. doi: 10.1083/jcb.200502088. J Cell Biol. 2005. PMID: 15967811 Free PMC article.
-
P-bodies and stress granules: possible roles in the control of translation and mRNA degradation.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a012286. doi: 10.1101/cshperspect.a012286. Cold Spring Harb Perspect Biol. 2012. PMID: 22763747 Free PMC article. Review.
-
Mammalian stress granules and P bodies at a glance.J Cell Sci. 2020 Sep 1;133(16):jcs242487. doi: 10.1242/jcs.242487. J Cell Sci. 2020. PMID: 32873715 Free PMC article. Review.
Cited by
-
Drosophila Clueless ribonucleoprotein particles display novel dynamics that rely on the availability of functional protein and polysome equilibrium.bioRxiv [Preprint]. 2024 Aug 22:2024.08.21.609023. doi: 10.1101/2024.08.21.609023. bioRxiv. 2024. PMID: 39229069 Free PMC article. Preprint.
-
Unveiling the Role of SlRNC1 in Chloroplast Development and Global Gene Regulation in Tomato Plants.Int J Mol Sci. 2024 Jun 24;25(13):6898. doi: 10.3390/ijms25136898. Int J Mol Sci. 2024. PMID: 39000008 Free PMC article.
-
Landscape of biomolecular condensates in heat stress responses.Front Plant Sci. 2022 Oct 6;13:1032045. doi: 10.3389/fpls.2022.1032045. eCollection 2022. Front Plant Sci. 2022. PMID: 36311142 Free PMC article. Review.
-
Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1.Viruses. 2023 Feb 6;15(2):449. doi: 10.3390/v15020449. Viruses. 2023. PMID: 36851663 Free PMC article. Review.
-
MERS-CoV nsp1 impairs the cellular metabolic processes by selectively downregulating mRNAs in a novel granules.Virulence. 2022 Dec;13(1):355-369. doi: 10.1080/21505594.2022.2032928. Virulence. 2022. PMID: 35129074 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
