Adult Ube3a Gene Reinstatement Restores the Electrophysiological Deficits of Prefrontal Cortex Layer 5 Neurons in a Mouse Model of Angelman Syndrome
- PMID: 30082419
- PMCID: PMC6596147
- DOI: 10.1523/JNEUROSCI.0083-18.2018
Adult Ube3a Gene Reinstatement Restores the Electrophysiological Deficits of Prefrontal Cortex Layer 5 Neurons in a Mouse Model of Angelman Syndrome
Abstract
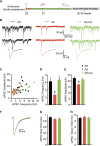
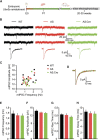
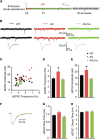
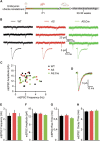
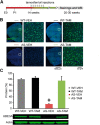
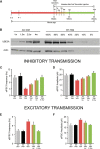
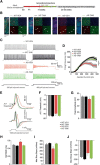
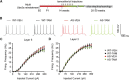
E3 ubiquitin ligase (UBE3A) levels in the brain need to be tightly regulated, as loss of functional UBE3A protein is responsible for the severe neurodevelopmental disorder Angelman syndrome (AS), whereas increased activity of UBE3A is associated with nonsyndromic autism. Given the role of mPFC in neurodevelopmental disorders including autism, we aimed to identify the functional changes resulting from loss of UBE3A in infralimbic and prelimbic mPFC areas in a mouse model of AS. Whole-cell recordings from layer 5 mPFC pyramidal neurons obtained in brain slices from adult mice of both sexes revealed that loss of UBE3A results in a strong decrease of spontaneous inhibitory transmission and increase of spontaneous excitatory transmission potentially leading to a marked excitation/inhibition imbalance. Additionally, we found that loss of UBE3A led to decreased excitability and increased threshold for action potential of layer 5 fast spiking interneurons without significantly affecting the excitability of pyramidal neurons. Because we previously showed that AS mouse behavioral phenotypes are reversible upon Ube3a gene reactivation during a restricted period of early postnatal development, we investigated whether Ube3a gene reactivation in a fully mature brain could reverse any of the identified physiological deficits. In contrast to our previously reported behavioral findings, restoring UBE3A levels in adult animals fully rescued all the identified physiological deficits of mPFC neurons. Moreover, the kinetics of reversing these synaptic deficits closely followed the reinstatement of UBE3A protein level. Together, these findings show a striking dissociation between the rescue of behavioral and physiological deficits.SIGNIFICANCE STATEMENT Here we describe significant physiological deficits in the mPFC of an Angelman syndrome mouse model. We found a marked change in excitatory/inhibitory balance, as well as decreased excitability of fast spiking interneurons. A promising treatment strategy for Angelman syndrome is aimed at restoring UBE3A expression by activating the paternal UBE3A gene. Here we find that the physiological changes in the mPFC are fully reversible upon gene reactivation, even when the brain is fully mature. This indicates that there is no critical developmental window for reversing the identified physiological deficits in mPFC.
Keywords: UBE3A; autistic disorder; disease models; ion channel; prefrontal cortex; synaptic transmission.
Copyright © 2018 the authors 0270-6474/18/388011-20$15.00/0.
Figures


Similar articles
-
Ube3a loss increases excitability and blunts orientation tuning in the visual cortex of Angelman syndrome model mice.J Neurophysiol. 2017 Jul 1;118(1):634-646. doi: 10.1152/jn.00618.2016. Epub 2017 May 3. J Neurophysiol. 2017. PMID: 28468997 Free PMC article.
-
Assessing the requirements of prenatal UBE3A expression for rescue of behavioral phenotypes in a mouse model for Angelman syndrome.Mol Autism. 2020 Sep 18;11(1):70. doi: 10.1186/s13229-020-00376-9. Mol Autism. 2020. PMID: 32948244 Free PMC article.
-
Enhanced Operant Extinction and Prefrontal Excitability in a Mouse Model of Angelman Syndrome.J Neurosci. 2018 Mar 14;38(11):2671-2682. doi: 10.1523/JNEUROSCI.2828-17.2018. Epub 2018 Feb 5. J Neurosci. 2018. PMID: 29431654 Free PMC article.
-
Angelman Syndrome: From Mouse Models to Therapy.Neuroscience. 2020 Oct 1;445:172-189. doi: 10.1016/j.neuroscience.2020.02.017. Epub 2020 Feb 21. Neuroscience. 2020. PMID: 32088294 Review.
-
Towards an understanding of Angelman syndrome in mice studies.J Neurosci Res. 2020 Jun;98(6):1162-1173. doi: 10.1002/jnr.24576. Epub 2019 Dec 22. J Neurosci Res. 2020. PMID: 31867793 Review.
Cited by
-
Daily Oscillation of the Excitation-Inhibition Balance in Visual Cortical Circuits.Neuron. 2020 Feb 19;105(4):621-629.e4. doi: 10.1016/j.neuron.2019.11.011. Epub 2019 Dec 9. Neuron. 2020. PMID: 31831331 Free PMC article.
-
Electrophysiological Characterization of Regular and Burst Firing Pyramidal Neurons of the Dorsal Subiculum in an Angelman Syndrome Mouse Model.Front Cell Neurosci. 2021 Aug 26;15:670998. doi: 10.3389/fncel.2021.670998. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34512263 Free PMC article.
-
Regional and cellular organization of the autism-associated protein UBE3A/E6AP and its antisense transcript in the brain of the developing rhesus monkey.Front Neuroanat. 2024 May 30;18:1410791. doi: 10.3389/fnana.2024.1410791. eCollection 2024. Front Neuroanat. 2024. PMID: 38873093 Free PMC article.
-
The Hippocampal Response to Acute Corticosterone Elevation Is Altered in a Mouse Model for Angelman Syndrome.Int J Mol Sci. 2022 Dec 24;24(1):303. doi: 10.3390/ijms24010303. Int J Mol Sci. 2022. PMID: 36613751 Free PMC article.
-
Stem cell models of Angelman syndrome.Front Cell Dev Biol. 2023 Oct 19;11:1274040. doi: 10.3389/fcell.2023.1274040. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37928900 Free PMC article. Review.
References
-
- Aghakhanyan G, Bonanni P, Randazzo G, Nappi S, Tessarotto F, De Martin L, Frijia F, De Marchi D, De Masi F, Kuppers B, Lombardo F, Caramella D, Montanaro D (2016) From cortical and subcortical grey matter abnormalities to neurobehavioral phenotype of Angelman syndrome: a voxel-based morphometry study. PLoS One 11:e0162817. 10.1371/journal.pone.0162817 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
