TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice
- PMID: 30070769
- PMCID: PMC6800140
- DOI: 10.1111/1755-5922.12462
TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice
Abstract
Introduction: Administration of cholic acid, or its synthetic derivative, 6-alpha-ethyl-23(S)-methylcholic acid (INT-777), activates the membrane GPCR, TGR5, influences whole body metabolism, reduces atherosclerosis, and benefits the cardiovascular physiology in mice. Direct effects of TGR5 agonists, and the role for TGR5, on myocardial cell biology and stress response are unknown.
Methods: Mice were fed chow supplemented with 0.5% cholic acid (CA) or 0.025% INT-777, a specific TGR5 agonist, or regular chow for 3 weeks. Anthropometric, biochemical, physiologic (electrocardiography and echocardiography), and molecular analysis was performed at baseline. CA and INT-777 fed mice were challenged with acute exercise-induced stress, acute catecholamine-induced stress, and hemodynamic stress induced by transverse aortic constriction (TAC) for a period of 8 weeks. In separate experiments, mice born with constitutive deletion of TGR5 in cardiomyocytes (CM-TGR5del ) were exposed to exercise, inotropic, and TAC-induced stress.
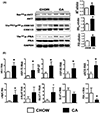
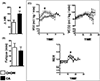
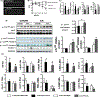
Results: Administration of CA and INT-777 supplemented diets upregulated TGR5 expression and activated Akt, PKA, and ERK1/2 in the heart. CA and INT-777 fed mice showed improved exercise tolerance, improved sensitivity to catecholamine and attenuation in pathologic remodeling of the heart under hemodynamic stress. In contrast, CM-TGR5del showed poor response to exercise and catecholamine challenge as well as higher mortality and signs of accelerated cardiomyopathy under hemodynamic stress.
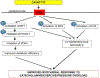
Conclusions: Bile acids, specifically TGR5 agonists, induce cytoprotective changes in the heart and improve myocardial response to physiologic, inotropic, and hemodynamic stress in mice. TGR5 plays a critical role in myocardial adaptability, and TGR5 activation may represent a potentially attractive treatment option in heart failure.
Keywords: INT-777; TGR5; cholic acid; myocardial adaptation; preconditioning.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures


Similar articles
-
Bile Acid G Protein-Coupled Membrane Receptor TGR5 Modulates Aquaporin 2-Mediated Water Homeostasis.J Am Soc Nephrol. 2018 Nov;29(11):2658-2670. doi: 10.1681/ASN.2018030271. Epub 2018 Oct 10. J Am Soc Nephrol. 2018. PMID: 30305310 Free PMC article.
-
Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration.Gastroenterology. 2020 Sep;159(3):956-968.e8. doi: 10.1053/j.gastro.2020.05.067. Epub 2020 May 30. Gastroenterology. 2020. PMID: 32485177
-
FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity.J Am Soc Nephrol. 2018 Jan;29(1):118-137. doi: 10.1681/ASN.2017020222. Epub 2017 Oct 31. J Am Soc Nephrol. 2018. PMID: 29089371 Free PMC article.
-
Bile acid receptors in the biliary tree: TGR5 in physiology and disease.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1319-1325. doi: 10.1016/j.bbadis.2017.08.021. Epub 2017 Aug 25. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28844960 Review.
-
Nitroxyl (HNO) for treatment of acute heart failure.Curr Heart Fail Rep. 2014 Sep;11(3):227-35. doi: 10.1007/s11897-014-0210-z. Curr Heart Fail Rep. 2014. PMID: 24980211 Review.
Cited by
-
Combined analysis of cecal microbiota and metabolomics reveals the intervention mechanism of Dayuan Yin in acute lung injury.Front Pharmacol. 2024 Sep 10;15:1436017. doi: 10.3389/fphar.2024.1436017. eCollection 2024. Front Pharmacol. 2024. PMID: 39318776 Free PMC article.
-
Heart Failure: a Punch from the Gut.Curr Heart Fail Rep. 2024 Apr;21(2):73-80. doi: 10.1007/s11897-024-00648-y. Epub 2024 Feb 1. Curr Heart Fail Rep. 2024. PMID: 38300390 Free PMC article. Review.
-
Bile acids and salt-sensitive hypertension: a role of the gut-liver axis.Am J Physiol Heart Circ Physiol. 2022 Apr 1;322(4):H636-H646. doi: 10.1152/ajpheart.00027.2022. Epub 2022 Mar 4. Am J Physiol Heart Circ Physiol. 2022. PMID: 35245132 Free PMC article. Review.
-
Gut microbiome and its meta-omics perspectives: profound implications for cardiovascular diseases.Gut Microbes. 2021 Jan-Dec;13(1):1936379. doi: 10.1080/19490976.2021.1936379. Gut Microbes. 2021. PMID: 34170211 Free PMC article. Review.
-
The Role of Intestinal Flora and Its Metabolites in Heart Failure.Infect Drug Resist. 2023 Jan 5;16:51-64. doi: 10.2147/IDR.S390582. eCollection 2023. Infect Drug Resist. 2023. PMID: 36636378 Free PMC article. Review.
References
-
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. - PubMed
-
- Groen AK. The emerging role of bile acids as integrators of intermediary metabolism. J Hepatol. 2006;45(2):337–338. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
