The role of JNK phosphorylation as a molecular target to enhance adenovirus replication, oncolysis and cancer therapeutic efficacy
- PMID: 30067431
- PMCID: PMC6301809
- DOI: 10.1080/15384047.2018.1491503
The role of JNK phosphorylation as a molecular target to enhance adenovirus replication, oncolysis and cancer therapeutic efficacy
Abstract
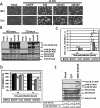
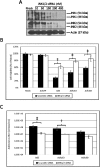
Oncolytic adenoviruses (Ads) are cancer selective tumoricidal agents; however their mechanism of Ad-mediated cancer cell lysis, or oncolysis, remains undefined. This report focuses upon the autophagy mediator c-JUN n-terminal kinase (JNK) and its effects upon Ad oncolysis and replication. Previously, E1b-deleted Ads have been used to treat several hundred cancer patients with limited clinical efficacy. We hypothesize that by studying the potential interactions between E1b and JNK, mechanisms to improve oncolytic Ad design and cancer therapeutic efficacy may be elucidated. To test this hypothesis, E1b was selectively deleted from the Ad genome. These studies indicated that Ads encoding E1b induced JNK phosphorylation predominately occurred via E1b-19K. The expression of another crucial Ad gene E1a was then overexpressed by the CMV promoter via the replication competent Ad vector Adhz69; these data indicated that E1A also induced JNK phosphorylation. To assess the effects of host cell JNK expression upon Ad oncolysis and replication, siRNA targeting JNK1 and JNK2 (JNK1/2) were utilized. The oncolysis and replication of the E1b-19K wild-type Ads Ad5 and Adhz63 were significantly attenuated following JNK1/2 siRNA transfection. However the oncolytic effects and replication of the E1b-19K deleted Ad Adhz60 were not altered by JNK1/2 siRNA transfection, further implicating the crucial role of E1b-19K for Ad oncolysis and replication via JNK phosphorylation. This study has demonstrated for the first time that JNK is an intriguing molecular marker associated with enhanced Ad virotherapy efficacy, influencing future Ad vector design.
Keywords: E1a; E1b; JNK; autophagy; lung cancer; molecular targets; oncolysis.
Figures



Similar articles
-
The antitumor efficacy of IL-24 mediated by E1A and E1B triple regulated oncolytic adenovirus.Cancer Biol Ther. 2010 Aug 1;10(3):242-50. doi: 10.4161/cbt.10.3.12308. Epub 2010 Aug 10. Cancer Biol Ther. 2010. PMID: 20574150
-
Dissecting the roles of E1A and E1B in adenoviral replication and RCAd-enhanced RDAd transduction efficacy on tumor cells.Cancer Biol Ther. 2014 Oct;15(10):1358-66. doi: 10.4161/cbt.29842. Epub 2014 Jul 14. Cancer Biol Ther. 2014. PMID: 25019940 Free PMC article.
-
E1A, E1B double-restricted adenovirus with RGD-fiber modification exhibits enhanced oncolysis for CAR-deficient biliary cancers.Clin Cancer Res. 2007 May 15;13(10):3043-50. doi: 10.1158/1078-0432.CCR-06-2103. Clin Cancer Res. 2007. PMID: 17505007
-
Oncolytic Replication of E1b-Deleted Adenoviruses.Viruses. 2015 Nov 6;7(11):5767-79. doi: 10.3390/v7112905. Viruses. 2015. PMID: 26561828 Free PMC article. Review.
-
A compendium of adenovirus genetic modifications for enhanced replication, oncolysis, and tumor immunosurveillance in cancer therapy.Gene. 2018 Dec 30;679:11-18. doi: 10.1016/j.gene.2018.08.069. Epub 2018 Aug 29. Gene. 2018. PMID: 30171937 Review.
Cited by
-
Nanoparticles augment the therapeutic window of RT and immunotherapy for treating cancers: pivotal role of autophagy.Theranostics. 2023 Jan 1;13(1):40-58. doi: 10.7150/thno.77233. eCollection 2023. Theranostics. 2023. PMID: 36593951 Free PMC article. Review.
-
Autophagy-related signaling pathways are involved in cancer (Review).Exp Ther Med. 2021 Jul;22(1):710. doi: 10.3892/etm.2021.10142. Epub 2021 May 3. Exp Ther Med. 2021. PMID: 34007319 Free PMC article. Review.
-
Bone and Soft-Tissue Sarcoma: A New Target for Telomerase-Specific Oncolytic Virotherapy.Cancers (Basel). 2020 Feb 18;12(2):478. doi: 10.3390/cancers12020478. Cancers (Basel). 2020. PMID: 32085583 Free PMC article. Review.
References
-
- Bischoff, J. R., Kirn D.H, Williams A., Heise C., Horn S., Muna M., Ng L., Nye J. A., Sampson-Johannes A., Fattaey A., McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996; 274(5286):373–376. - PubMed
-
- Khuri, F. R., Nemunaitis J., Ganly I., Arseneau J., Tannock I. F., Romel. L., Gore M., Ironside J., MacDougall R. H., Heise C., Randlev B., Gillenwater A. M., Bruso P., Kaye S. B., Hong W. K., Kirn D. H. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–885. doi:10.1038/78638. - DOI - PubMed
-
- Nemunaitis J., Cunningham C., Buchanan A., Blackburn A., Edelman G., Maples P., Netto G., Tong A., Randlev B., Olson S., Kirn D. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8(10):746–759. doi:10.1038/sj.gt.3301424. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
