Past and Present Perspectives on β-Lactamases
- PMID: 30061284
- PMCID: PMC6153792
- DOI: 10.1128/AAC.01076-18
Past and Present Perspectives on β-Lactamases
Abstract
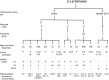
β-Lactamases, the major resistance determinant for β-lactam antibiotics in Gram-negative bacteria, are ancient enzymes whose origins can be traced back millions of years ago. These well-studied enzymes, currently numbering almost 2,800 unique proteins, initially emerged from environmental sources, most likely to protect a producing bacterium from attack by naturally occurring β-lactams. Their ancestors were presumably penicillin-binding proteins that share sequence homology with β-lactamases possessing an active-site serine. Metallo-β-lactamases also exist, with one or two catalytically functional zinc ions. Although penicillinases in Gram-positive bacteria were reported shortly after penicillin was introduced clinically, transmissible β-lactamases that could hydrolyze recently approved cephalosporins, monobactams, and carbapenems later became important in Gram-negative pathogens. Nomenclature is based on one of two major systems. Originally, functional classifications were used, based on substrate and inhibitor profiles. A later scheme classifies β-lactamases according to amino acid sequences, resulting in class A, B, C, and D enzymes. A more recent nomenclature combines the molecular and biochemical classifications into 17 functional groups that describe most β-lactamases. Some of the most problematic enzymes in the clinical community include extended-spectrum β-lactamases (ESBLs) and the serine and metallo-carbapenemases, all of which are at least partially addressed with new β-lactamase inhibitor combinations. New enzyme variants continue to be described, partly because of the ease of obtaining sequence data from whole-genome sequencing studies. Often, these new enzymes are devoid of any phenotypic descriptions, making it more difficult for clinicians and antibiotic researchers to address new challenges that may be posed by unusual β-lactamases.
Keywords: ESBL; MBL; carbapenemase; cephalosporinase; penicillinase; β-lactam; β-lactamase.
Copyright © 2018 American Society for Microbiology.
Figures
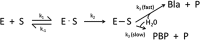

Similar articles
-
Potential involvement of beta-lactamase homologous proteins in resistance to beta-lactam antibiotics in gram-negative bacteria of the ESKAPEE group.BMC Genomics. 2024 May 22;25(1):508. doi: 10.1186/s12864-024-10410-2. BMC Genomics. 2024. PMID: 38778284 Free PMC article.
-
Class D β-lactamases: a reappraisal after five decades.Acc Chem Res. 2013 Nov 19;46(11):2407-15. doi: 10.1021/ar300327a. Epub 2013 Jul 31. Acc Chem Res. 2013. PMID: 23902256 Free PMC article. Review.
-
β-lactamase-mediated resistance: a biochemical, epidemiological and genetic overview.Curr Pharm Des. 2013;19(2):164-208. Curr Pharm Des. 2013. PMID: 22894615 Review.
-
Epidemiology of β-Lactamase-Producing Pathogens.Clin Microbiol Rev. 2020 Feb 26;33(2):e00047-19. doi: 10.1128/CMR.00047-19. Print 2020 Mar 18. Clin Microbiol Rev. 2020. PMID: 32102899 Free PMC article. Review.
-
Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens.Int J Med Microbiol. 2010 Aug;300(6):371-9. doi: 10.1016/j.ijmm.2010.04.005. Epub 2010 May 27. Int J Med Microbiol. 2010. PMID: 20537585 Review.
Cited by
-
The role of adjuvants in overcoming antibacterial resistance due to enzymatic drug modification.RSC Med Chem. 2022 Sep 22;13(11):1276-1299. doi: 10.1039/d2md00263a. eCollection 2022 Nov 16. RSC Med Chem. 2022. PMID: 36439977 Free PMC article. Review.
-
Bacterial Genome Wide Association Studies (bGWAS) and Transcriptomics Identifies Cryptic Antimicrobial Resistance Mechanisms in Acinetobacter baumannii.Front Public Health. 2020 Sep 2;8:451. doi: 10.3389/fpubh.2020.00451. eCollection 2020. Front Public Health. 2020. PMID: 33014966 Free PMC article.
-
Evaluation of In Vitro Activity of Double-Carbapenem Combinations against KPC-2-, OXA-48- and NDM-Producing Escherichia coli and Klebsiella pneumoniae.Antibiotics (Basel). 2022 Nov 17;11(11):1646. doi: 10.3390/antibiotics11111646. Antibiotics (Basel). 2022. PMID: 36421290 Free PMC article.
-
Thermostable β-Lactamase Mutant with Its Active Site Conjugated with Fluorescein for Efficient β-Lactam Antibiotic Detection.ACS Omega. 2019 Nov 27;4(24):20493-20502. doi: 10.1021/acsomega.9b02211. eCollection 2019 Dec 10. ACS Omega. 2019. PMID: 31858033 Free PMC article.
-
Phenotypic and Molecular Epidemiology of ESBL-, AmpC-, and Carbapenemase-Producing Escherichia coli in Northern and Eastern Europe.Front Microbiol. 2019 Nov 22;10:2465. doi: 10.3389/fmicb.2019.02465. eCollection 2019. Front Microbiol. 2019. PMID: 31824436 Free PMC article.
References
-
- Fisher JF, Knowles JR. 1978. Bacterial resistance to β-lactams: the β-lactamases. Ann Rep Med Chem 13:239–248. doi:10.1016/S0065-7743(08)60628-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

