Obesity-mediated regulation of cardiac protein acetylation: parallel analysis of total and acetylated proteins via TMT-tagged mass spectrometry
- PMID: 30061171
- PMCID: PMC6127670
- DOI: 10.1042/BSR20180721
Obesity-mediated regulation of cardiac protein acetylation: parallel analysis of total and acetylated proteins via TMT-tagged mass spectrometry
Abstract
Lysine residues undergo diverse and reversible post-translational modifications (PTMs). Lysine acetylation has traditionally been studied in the epigenetic regulation of nucleosomal histones that provides an important mechanism for regulating gene expression. Histone acetylation plays a key role in cardiac remodeling and function. However, recent studies have shown that thousands of proteins can be acetylated at multiple acetylation sites, suggesting the acetylome rivals the kinome as a PTM. Based on this, we examined the impact of obesity on protein lysine acetylation in the left ventricle (LV) of male c57BL/6J mice. We reported that obesity significantly increased heart enlargement and fibrosis. Moreover, immunoblot analysis demonstrated that lysine acetylation was markedly altered with obesity and that this phenomenon was cardiac tissue specific. Mass spectral analysis identified 2515 proteins, of which 65 were significantly impacted by obesity. Ingenuity Pathway Analysis® (IPA) further demonstrated that these proteins were involved in metabolic dysfunction and cardiac remodeling. In addition to total protein, 189 proteins were acetylated, 14 of which were significantly impacted by obesity. IPA identified the Cardiovascular Disease Pathway as significantly regulated by obesity. This network included aconitate hydratase 2 (ACO2), and dihydrolipoyl dehydrogenase (DLD), in which acetylation was significantly increased by obesity. These proteins are known to regulate cardiac function yet, the impact for ACO2 and DLD acetylation remains unclear. Combined, these findings suggest a critical role for cardiac acetylation in obesity-mediated remodeling; this has the potential to elucidate novel targets that regulate cardiac pathology.
Keywords: acetylation/deacetylation; muscle contraction; muscle metabolism; myocardial remodeling; proteomics.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



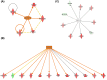
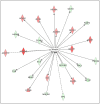
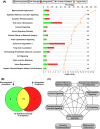
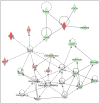
Similar articles
-
Global proteomic analysis of lysine acetylation in zebrafish (Danio rerio) embryos.Electrophoresis. 2016 Dec;37(23-24):3137-3145. doi: 10.1002/elps.201600210. Epub 2016 Nov 2. Electrophoresis. 2016. PMID: 27696471
-
Proteome-wide analysis of lysine acetylation in adult Schistosoma japonicum worm.J Proteomics. 2016 Oct 4;148:202-12. doi: 10.1016/j.jprot.2016.08.008. Epub 2016 Aug 12. J Proteomics. 2016. PMID: 27535354
-
Proteome-wide lysine acetylation identification in developing rice (Oryza sativa) seeds and protein co-modification by acetylation, succinylation, ubiquitination, and phosphorylation.Biochim Biophys Acta Proteins Proteom. 2018 Mar;1866(3):451-463. doi: 10.1016/j.bbapap.2017.12.001. Epub 2017 Dec 5. Biochim Biophys Acta Proteins Proteom. 2018. PMID: 29313810
-
Acetylation in cardiovascular diseases: Molecular mechanisms and clinical implications.Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165836. doi: 10.1016/j.bbadis.2020.165836. Epub 2020 May 12. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32413386 Review.
-
Functions and mechanisms of non-histone protein acetylation.Nat Rev Mol Cell Biol. 2019 Mar;20(3):156-174. doi: 10.1038/s41580-018-0081-3. Nat Rev Mol Cell Biol. 2019. PMID: 30467427 Review.
Cited by
-
Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies.Signal Transduct Target Ther. 2023 May 27;8(1):220. doi: 10.1038/s41392-023-01439-y. Signal Transduct Target Ther. 2023. PMID: 37244925 Free PMC article. Review.
-
Quantitative proteomic analysis to identify differentially expressed proteins in the persistent atrial fibrillation using TMT coupled with nano-LC-MS/MS.Am J Transl Res. 2020 Sep 15;12(9):5032-5047. eCollection 2020. Am J Transl Res. 2020. PMID: 33042404 Free PMC article.
-
The role of acetylation in obesity-induced cardiac metabolic alterations.J Pharm Pharm Sci. 2024 Jul 23;27:13080. doi: 10.3389/jpps.2024.13080. eCollection 2024. J Pharm Pharm Sci. 2024. PMID: 39109269 Free PMC article. Review.
-
Landscape of heart proteome changes in a diet-induced obesity model.Sci Rep. 2019 Dec 2;9(1):18050. doi: 10.1038/s41598-019-54522-2. Sci Rep. 2019. PMID: 31792287 Free PMC article.
-
Disruption of Acetyl-Lysine Turnover in Muscle Mitochondria Promotes Insulin Resistance and Redox Stress without Overt Respiratory Dysfunction.Cell Metab. 2020 Jan 7;31(1):131-147.e11. doi: 10.1016/j.cmet.2019.11.003. Epub 2019 Dec 5. Cell Metab. 2020. PMID: 31813822 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

