GSK3-β promotes calpain-1-mediated desmin filament depolymerization and myofibril loss in atrophy
- PMID: 30061109
- PMCID: PMC6168250
- DOI: 10.1083/jcb.201802018
GSK3-β promotes calpain-1-mediated desmin filament depolymerization and myofibril loss in atrophy
Abstract
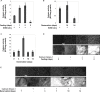
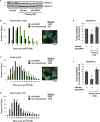
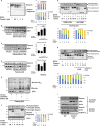
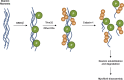
Myofibril breakdown is a fundamental cause of muscle wasting and inevitable sequel of aging and disease. We demonstrated that myofibril loss requires depolymerization of the desmin cytoskeleton, which is activated by phosphorylation. Here, we developed a mass spectrometry-based kinase-trap assay and identified glycogen synthase kinase 3-β (GSK3-β) as responsible for desmin phosphorylation. GSK3-β inhibition in mice prevented desmin phosphorylation and depolymerization and blocked atrophy upon fasting or denervation. Desmin was phosphorylated by GSK3-β 3 d after denervation, but depolymerized only 4 d later when cytosolic Ca2+ levels rose. Mass spectrometry analysis identified GSK3-β and the Ca2+-specific protease, calpain-1, bound to desmin and catalyzing its disassembly. Consistently, calpain-1 down-regulation prevented loss of phosphorylated desmin and blocked atrophy. Thus, phosphorylation of desmin filaments by GSK3-β is a key molecular event required for calpain-1-mediated depolymerization, and the subsequent myofibril destruction. Consequently, GSK3-β represents a novel drug target to prevent myofibril breakdown and atrophy.
© 2018 Aweida et al.
Figures


Similar articles
-
Role of calpains in promoting desmin filaments depolymerization and muscle atrophy.Biochim Biophys Acta Mol Cell Res. 2020 Oct;1867(10):118788. doi: 10.1016/j.bbamcr.2020.118788. Epub 2020 Jun 27. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32603758 Review.
-
Myofibril breakdown during atrophy is a delayed response requiring the transcription factor PAX4 and desmin depolymerization.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):E1375-E1384. doi: 10.1073/pnas.1612988114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096335 Free PMC article.
-
Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy.J Cell Biol. 2012 Aug 20;198(4):575-89. doi: 10.1083/jcb.201110067. J Cell Biol. 2012. PMID: 22908310 Free PMC article.
-
Sarcoplasmic and myofibril-bound calpains during storage of pork longissimus muscle: New insights on protein degradation.Food Chem. 2022 Mar 15;372:131347. doi: 10.1016/j.foodchem.2021.131347. Epub 2021 Oct 7. Food Chem. 2022. PMID: 34818749
-
New roles for desmin in the maintenance of muscle homeostasis.FEBS J. 2022 May;289(10):2755-2770. doi: 10.1111/febs.15864. Epub 2021 Apr 22. FEBS J. 2022. PMID: 33825342 Review.
Cited by
-
Calpain-mediated proteolysis of vimentin filaments is augmented in giant axonal neuropathy fibroblasts exposed to hypotonic stress.Front Cell Dev Biol. 2022 Nov 1;10:1008542. doi: 10.3389/fcell.2022.1008542. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393840 Free PMC article.
-
Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy.Int J Mol Sci. 2021 Aug 2;22(15):8310. doi: 10.3390/ijms22158310. Int J Mol Sci. 2021. PMID: 34361076 Free PMC article. Review.
-
Desmin aggrephagy in rat and human ischemic heart failure through PKCζ and GSK3β as upstream signaling pathways.Cell Death Discov. 2021 Jun 26;7(1):153. doi: 10.1038/s41420-021-00549-2. Cell Death Discov. 2021. PMID: 34226534 Free PMC article.
-
Molecular Mechanisms of Muscle Tone Impairment under Conditions of Real and Simulated Space Flight.Acta Naturae. 2021 Apr-Jun;13(2):85-97. doi: 10.32607/actanaturae.10953. Acta Naturae. 2021. PMID: 34377559 Free PMC article.
-
Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis.Nat Cell Biol. 2022 May;24(5):793-804. doi: 10.1038/s41556-022-00893-0. Epub 2022 Apr 25. Nat Cell Biol. 2022. PMID: 35469018 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

