Annexin A2 binds to vimentin and contributes to porcine reproductive and respiratory syndrome virus multiplication
- PMID: 30053894
- PMCID: PMC6064111
- DOI: 10.1186/s13567-018-0571-5
Annexin A2 binds to vimentin and contributes to porcine reproductive and respiratory syndrome virus multiplication
Erratum in
-
Correction to: Annexin A2 binds to vimentin and contributes to porcine reproductive and respiratory syndrome virus multiplication.Vet Res. 2018 Oct 5;49(1):103. doi: 10.1186/s13567-018-0595-x. Vet Res. 2018. PMID: 30290850 Free PMC article.
Abstract
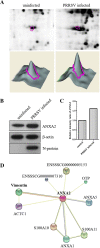
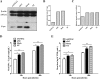
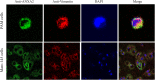
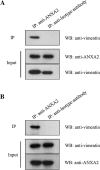
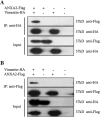
Porcine reproductive and respiratory syndrome virus (PRRSV) is an important globally distributed and highly contagious pathogen that has restricted cell tropism in vivo and in vitro. In the present study, we found that annexin A2 (ANXA2) is upregulated expressed in porcine alveolar macrophages infected with PRRSV. Additionally, PRRSV replication was significantly suppressed after reducing ANXA2 expression in Marc-145 cells using siRNA. Bioinformatics analysis indicated that ANXA2 may be relevant to vimentin, a cellular cytoskeleton component that is thought to be involved in the infectivity and replication of PRRSV. Co-immunoprecipitation assays and confocal analysis confirmed that ANXA2 interacts with vimentin, with further experiments indicating that the B domain (109-174 aa) of ANXA2 contributes to this interaction. Importantly, neither ANXA2 nor vimentin alone could bind to PRRSV and only in the presence of ANXA2 could vimentin interact with the N protein of PRRSV. No binding to the GP2, GP3, GP5, nor M proteins of PRRSV was observed. In conclusion, ANXA2 can interact with vimentin and enhance PRRSV growth. This contributes to the regulation of PRRSV replication in infected cells and may have implications for the future antiviral strategies.
Figures


Similar articles
-
The interaction between host Annexin A2 and viral Nsp9 is beneficial for replication of porcine reproductive and respiratory syndrome virus.Virus Res. 2014 Aug 30;189:106-13. doi: 10.1016/j.virusres.2014.05.015. Epub 2014 May 26. Virus Res. 2014. PMID: 24874199
-
Interleukin-2 enhancer binding factor 2 interacts with the nsp9 or nsp2 of porcine reproductive and respiratory syndrome virus and exerts negatively regulatory effect on the viral replication.Virol J. 2017 Jul 11;14(1):125. doi: 10.1186/s12985-017-0794-5. Virol J. 2017. PMID: 28693575 Free PMC article.
-
An intracellularly expressed Nsp9-specific nanobody in MARC-145 cells inhibits porcine reproductive and respiratory syndrome virus replication.Vet Microbiol. 2015 Dec 31;181(3-4):252-60. doi: 10.1016/j.vetmic.2015.10.021. Epub 2015 Oct 26. Vet Microbiol. 2015. PMID: 26525739
-
PRRSV receptors and their roles in virus infection.Arch Microbiol. 2015 May;197(4):503-12. doi: 10.1007/s00203-015-1088-1. Epub 2015 Feb 11. Arch Microbiol. 2015. PMID: 25666932 Review.
-
[Advances in Understanding of the Infection/Replication Mechanisms and Virulence Determinants of the Porcine Reproductive and Respiratory Syndrome Virus].Bing Du Xue Bao. 2015 Sep;31(5):585-92. Bing Du Xue Bao. 2015. PMID: 26738300 Review. Chinese.
Cited by
-
Mechanical and Non-Mechanical Functions of Filamentous and Non-Filamentous Vimentin.Bioessays. 2020 Nov;42(11):e2000078. doi: 10.1002/bies.202000078. Epub 2020 Sep 6. Bioessays. 2020. PMID: 32893352 Free PMC article. Review.
-
Extracellular vimentin as a target against SARS-CoV-2 host cell invasion.bioRxiv [Preprint]. 2021 Mar 18:2021.01.08.425793. doi: 10.1101/2021.01.08.425793. bioRxiv. 2021. Update in: Small. 2022 Feb;18(6):e2105640. doi: 10.1002/smll.202105640. PMID: 33442680 Free PMC article. Updated. Preprint.
-
Extracellular Vimentin as a Target Against SARS-CoV-2 Host Cell Invasion.Small. 2022 Feb;18(6):e2105640. doi: 10.1002/smll.202105640. Epub 2021 Dec 5. Small. 2022. PMID: 34866333 Free PMC article.
-
FADD promotes type I interferon production to suppress porcine reproductive and respiratory syndrome virus infection.Front Vet Sci. 2024 Apr 8;11:1380144. doi: 10.3389/fvets.2024.1380144. eCollection 2024. Front Vet Sci. 2024. PMID: 38650851 Free PMC article.
-
miR-541-3p Promoted Porcine Reproductive and Respiratory Syndrome Virus 2 (PRRSV-2) Replication by Targeting Interferon Regulatory Factor 7.Viruses. 2022 Jan 11;14(1):126. doi: 10.3390/v14010126. Viruses. 2022. PMID: 35062330 Free PMC article.
References
-
- Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 31270045/National Natural Science Foundation of China/International
- 2016RAQXJ142/Foundation for Science and Technology Innovative Talents of Harbin/International
- JC2017010/Heilongjiang Natural Science Fund for Distinguished Young Scholars/International
- Y2017JC16/Foundation for Basic Scientific Research of Central Public Research Institutes/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

