Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. A Cochrane Rapid Review
- PMID: 30036453
- PMCID: PMC6513246
- DOI: 10.1002/14651858.CD012838.pub2
Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. A Cochrane Rapid Review
Abstract
Background: The use of systemic immunotherapy targets is emerging as an important treatment option for metastatic urothelial carcinoma, particularly for patients who cannot tolerate or who fail cisplatin-based chemotherapy. One such target is the inhibition of the checkpoint protein programmed cell death-1 (PD-1) receptor and its ligand (PD-L1) by monoclonal antibodies.
Objectives: To assess the effects of pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy.
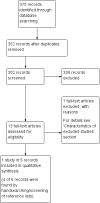
Search methods: We performed a Cochrane Rapid Review, limiting our search to published studies in the English language. We searched databases of the medical literature, including the Cochrane Central Register of Controlled Trials and MEDLINE, as well as trial registries including ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). Our search extended from January 2000 to June 2018.
Selection criteria: We included randomised controlled trials except cross-over trials and cluster randomised trials. We excluded all other study designs. Participants included had locally advanced or metastatic urothelial carcinoma of the bladder, with disease progression during or following platinum-containing chemotherapy (synonymous with second-/third-/fourth-line therapy). This review focused on pembrolizumab (synonyms: MK-3475, lambrolizumab, Keytruda).
Data collection and analysis: Two review authors independently classified and abstracted data from the included study. The certainty of evidence was rated according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
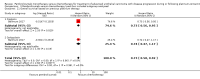
Main results: We identified one randomised controlled trial that included 542 participants, which compared the use of pembrolizumab monotherapy versus chemotherapy for the treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. Results were reported after a median follow-up of 14.1 months (range 9.9 to 22.1 months).Primary outcomesPembrolizumab probably reduces the risk of death from any cause (hazard ratio (HR) 0.73, 95% confidence interval (CI) 0.59 to 0.90; moderate certainty evidence). This corresponds to 115 fewer deaths (191 fewer to 38 fewer) per 1000 participants with pembrolizumab at 12 months. We downgraded the certainty of evidence one level for imprecision.Pembrolizumab may slightly improve quality of life (change from baseline to week 15 assessed with the Core Quality of Life Questionnaire; higher value reflects better quality of life; scale 0 to 100) with a mean difference (MD) of 9.05, 95% CI 4.61 to 13.50; low certainty evidence). We downgraded the certainty of evidence two levels for study limitations and imprecision.Secondary outcomesPembrolizumab may have little or no effect on disease progression (HR 0.98, 95% CI 0.81 to 1.19; low certainty evidence). This corresponds to three fewer patients (42 fewer to 24 more) whose disease progressed per 1000 participants at 12 months. We downgraded the certainty of evidence two levels for study limitations and imprecision.Pembrolizumab probably improves treatment response (based on complete or partial radiologic response) with a risk ratio (RR) of 1.85, 95% CI 1.24 to 2.77; moderate certainty evidence). This corresponds to 97 more respondents (27 more to 202 more) per 1000 participants with pembrolizumab. We downgraded the certainty of evidence one level for imprecision.Pembrolizumab may have little or no effect on treatment-related mortality (RR 0.96, 95% CI 0.24 to 3.79; low certainty evidence). This corresponds to one fewer (12 fewer to 44 more) treatment-related deaths per 1000 participants with pembrolizumab. We downgraded the certainty of evidence two levels for study limitations and imprecision.Pembrolizumab may have little or no effect on discontinuations due to adverse events (RR 0.66, 95% CI 0.39 to 1.10). This corresponds to 54 fewer discontinuations per 1000 participants (95% CI 79 fewer to 7 more). We downgraded the certainty of evidence for study limitations and imprecision.Pembrolizumab may reduce serious adverse events (RR 0.83, 95 CI 0.72 to 0.97; low certainty evidence). This corresponds to 107 fewer serious averse events per 1000 participants (95% CI 19 fewer to 176 fewer). We downgraded two levels for study limitations and imprecision.
Authors' conclusions: The use of pembrolizumab in men with advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy probably improves overall survival when compared with chemotherapy alone. At 12 months follow-up about 70% of those in the chemotherapy group had died, compared with 59% of those treated with pembrolizumab. We are very uncertain about the effects of pembolizumab on quality of life. Pembolizumab may also improve treatment response rates, and reduce the risk of serious adverse events, but may make little or no difference to discontinuations of treatment due to adverse events. These conclusions are based on a single trial that was sponsored by the producer of pembrolizumab.
Conflict of interest statement
VN: none.
AK: none.
PD: none.
NS: none.
MR: none.
CB: none.
NP: none.
JHJ: none.
ECH: none
GG: none.
FK: none.
Figures


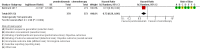

























Update of
- doi: 10.1002/14651858.CD012838
Similar articles
-
Immunotherapy for advanced or metastatic urothelial carcinoma.Cochrane Database Syst Rev. 2023 Oct 9;10(10):CD013774. doi: 10.1002/14651858.CD013774.pub2. Cochrane Database Syst Rev. 2023. PMID: 37811690 Free PMC article. Review.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Early versus deferred standard androgen suppression therapy for advanced hormone-sensitive prostate cancer.Cochrane Database Syst Rev. 2019 Jun 11;6(6):CD003506. doi: 10.1002/14651858.CD003506.pub2. Cochrane Database Syst Rev. 2019. PMID: 31194882 Free PMC article.
-
Immune checkpoint inhibitors plus platinum-based chemotherapy compared to platinum-based chemotherapy with or without bevacizumab for first-line treatment of older people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2024 Aug 13;8(8):CD015495. doi: 10.1002/14651858.CD015495. Cochrane Database Syst Rev. 2024. PMID: 39136258 Review.
Cited by
-
Immunotherapy for advanced or metastatic urothelial carcinoma.Cochrane Database Syst Rev. 2023 Oct 9;10(10):CD013774. doi: 10.1002/14651858.CD013774.pub2. Cochrane Database Syst Rev. 2023. PMID: 37811690 Free PMC article. Review.
-
Radiotherapy/Chemotherapy-Immunotherapy for Cancer Management: From Mechanisms to Clinical Implications.Oxid Med Cell Longev. 2023 Feb 2;2023:7530794. doi: 10.1155/2023/7530794. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36778203 Free PMC article. Review.
-
The expression pattern of Immune checkpoints after chemo/radiotherapy in the tumor microenvironment.Front Immunol. 2022 Jul 28;13:938063. doi: 10.3389/fimmu.2022.938063. eCollection 2022. Front Immunol. 2022. PMID: 35967381 Free PMC article. Review.
-
Misconduct in research integrity: Assessment the quality of systematic reviews in Cochrane urological cancer review group.Turk J Urol. 2021 Sep;47(5):392-419. doi: 10.5152/tud.2021.21038. Turk J Urol. 2021. PMID: 35118979 Free PMC article.
-
Current status of first-line therapy, anti-angiogenic therapy and its combinations of other agents for unresectable hepatocellular carcinoma.World J Gastrointest Oncol. 2021 Dec 15;13(12):2038-2049. doi: 10.4251/wjgo.v13.i12.2038. World J Gastrointest Oncol. 2021. PMID: 35070040 Free PMC article. Review.
References
References to studies included in this review
Bellmunt 2017 {published data only}
-
- Bellmunt J, Sonpavde G, Wit R, Choueiri TK, Siefker‐Radtke AO, Plimack ER, et al. KEYNOTE‐045: randomized phase 3 trial of pembrolizumab (MK‐3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer. Journal of Clinical Oncology 2015 [Epub ahead of print]; Vol. 33, issue 15 Suppl. [DOI: 10.1200/jco.2015.33.15_suppl.tps4571] - DOI
-
- Wit R, Bajorin DF, Bellmunt J, Fradet Y, Lee J‐L, Fong L. Health‐related quality of life (HRQoL) of pembrolizumab (pembro) vs chemotherapy (chemo) for previously treated advanced urothelial cancer (UC) in KEYNOTE‐045. Journal of Clinical Oncology 2017;35(15 Suppl):4530. [DOI: 10.1200/JCO.2017.35.15_suppl.4530] - DOI
-
- Merck Sharp, Dohme Corp. A study of pembrolizumab (MK‐3475) versus paclitaxel, docetaxel, or vinflunine for participants with advanced urothelial cancer (MK‐3475‐045/KEYNOTE‐045). clinicaltrials.gov/ct2/show/NCT02256436 (accessed 1 November 2017).
-
- Quinn D, Bellmunt J, Wit R, Vaughn DJ, Fradet Y, Lee JL, et al. Keynote‐045: open‐label, phase 3 study of pembrolizumab versus investigator's choice of paclitaxel, docetaxel, or vinflunine for previously treated advanced urothelial cancer. Asia‐Pacific Journal of Clinical Oncology 2017; Vol. 13, issue Suppl S1:35.
References to studies excluded from this review
Acerta 2017 {published data only}
-
- Acerta Pharma BV, Merck Sharp, Dohme Corp. Study of the combination of ACP‐196 and pembrolizumab in subjects with platinum resistant urothelial bladder cancer. clinicaltrials.gov/ct2/show/NCT02351739 (accessed 1 November 2017).
Alva 2016 {published data only}
-
- Alva A, Gschwendt J, Loriot Y, Bellmunt J, Feng D, Poehlein C, et al. KEYNOTE‐361: randomized phase III study of pembrolizumab with or without chemotherapy versus chemotherapy alone in advanced urothelial carcinoma. Journal for ImmunoTherapy of Cancer 2016; Vol. 4, issue Suppl 1.
Guo 2017 {published data only}
-
- Guo T, Feng C. Pembrolizumab for advanced urothelial carcinoma. New England Journal of Medicine 2017;376(23):2303‐4. - PubMed
Matthew 2015 {published data only}
-
- Galsky M, Hoosier Cancer Research Network and Merck Sharp & Dohme Corp. Testing the PD‐1 inhibitor pembrolizumab as maintenance therapy after initial chemotherapy in metastatic bladder cancer. clinicaltrials.gov/ct2/show/NCT02500121 accessed 1 November 2017.
Mitchell 2017 {published data only}
Powles 2017 {published data only}
-
- Powles T, Gschwend JE, Loriot Y, Bellmunt J, Geczi L, Vulsteke C, et al. Phase 3 KEYNOTE‐361 trial: pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. Journal of Clinical Oncology 2017 [Epub ahead of print]; Vol. 35, issue 15 Suppl. [DOI: 10.1200/JCO.2017.35.15_suppl.TPS4590] - DOI
Additional references
Bellmunt 2013
-
- Bellmunt J, Fougeray R, Rosenberg JE, Maase H, Schutz FA, Salhi Y, et al. Long‐term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum‐based chemotherapy. Annals of Oncology 2013;24(6):1466‐72. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation. Covidence. Version accessed 28 August 2017. Melbourne, Australia: Veritas Health Innovation, 2017.
EAU 2017
-
- Witjes JA, Compérat E, Cowan NC, Gakis G, Hernández V, Lebret T, et al. EAU guidelines on muscle‐invasive and metastatic bladder cancer. uroweb.org/guideline/bladder‐cancer‐muscle‐invasive‐and‐metastatic/ (accessed 1 October 2017).
Eisenhauer 2009
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009; Vol. 45, issue 2:228‐47. - PubMed
Endnote 2011 [Computer program]
-
- Thomson Reuters. EndNote X5. Version X5. Thomson Reuters, 2011.
Faraj 2015
GLOBOCAN 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013;Available from: http://globocan.iarc.fr:accessed 10/2017.
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 February 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 2015
-
- Gupta S, O'Donnell P, Plimack ER, Berger R, Montgomery B, Heath K, et al. Mp68‐11 a Phase 1B Study of Pembrolizumab (Pembro; Mk‐3475) for Advanced Urothelial Cancer. The Journal of Urology 2015;193(4):e861‐2.
Guyatt 2008
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011d
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodi 2016
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. - PMC - PubMed
IQWiG 2017
-
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Pembrolizumab (urothelial carcinoma) [Pembrolizumab (Urothelkarzinom)]. Institute for Quality and Efficiency in Health Care 2017; Vol. Dossierbewertung A17‐46, issue Version 1.0:3‐9.
Iwai 2002
Kim 2015
-
- Kim JW, Bellmunt J, Powles T, Loriot Y, Vogelzang MJ, Zambrano CC, et al. Clinical activity, safety, and biomarkers of MPDL3280A in metastatic urothelial bladder cancer: Additional analysis from phase IA study. Journal of Clinical Oncology 2015;33(7 Suppl):297.
Leitlinienprogramm Onkologie
-
- Leitlinienprogramm Onkologie, Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF. S3 ‐ Guideline for early detection, diagnosis, therapy and aftercare for bladder cancer [S3 ‐ Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms]. leitlinienprogramm‐onkologie.de/Harnblasenkarzinom.92.0.html (accessed 1 October 2017).
Liang 2017
-
- Liang F, Zhu J. Pembrolizumab for advanced urothelial carcinoma. New England Journal of Medicine 2017;376(23):2302. - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] - DOI - PMC - PubMed
Logothetis 1990
-
- Logothetis CJ, Dexeus FH, Finn L, Sella A, Amato RJ, Ayala AG, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. Journal of Clinical Oncology 1990;8(6):1050‐5. - PubMed
Morales 1976
-
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette‐Guerin in the treatment of superficial bladder tumors. The Journal of Urology 1976;116(2):180‐3. - PubMed
NCCN Guideline 2017
-
- Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, et al. Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2017;15(10):1240‐67. - PubMed
Park 2016
-
- Park JC, Hahn NM. Emerging role of immunotherapy in urothelial carcinoma—Future directions and novel therapies. Urologic Oncology: Seminars and Original Investigations 2016;34(12):566‐76. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] - PubMed
Plimack 2017
-
- Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE‐012): a non‐randomised, open‐label, phase 1b study. Lancet Oncology 2017;18(2):212‐20. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ribas 2015
-
- Ribas A. Releasing the brakes on cancer immunotherapy. New England Journal of Medicine 2015;373(16):1490–2. - PubMed
Rijnders 2017
-
- Rijnders M, Wit R, Boormans JL, Lolkema MP, Veldt AA. Systematic review of immune checkpoint inhibition in urological cancers. European Urology 2017;72(3):411‐23. - PubMed
Rosenberg 2016
-
- Rosenberg JE, Hoffman‐Censits J, Powles T, Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicentre, phase 2 trial. The Lancet 2016;387(10031):1909‐20. - PMC - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sharma 2015
-
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56‐61. - PubMed
Sharma 2016
Tierney 2007
von der Maase 2000
-
- Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. Journal of Clinical Oncology 2000;18(17):3068‐77. - PubMed
References to other published versions of this review
Narayan 2017
-
- Narayan V, Dahm P, Skoetz N, Risk MC, Bonfiorno C, Patel N, et al. Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. A Cochrane Rapid Review. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012838] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

