Cell-intrinsic and -extrinsic mechanisms promote cell-type-specific cytokinetic diversity
- PMID: 30028292
- PMCID: PMC6054530
- DOI: 10.7554/eLife.36204
Cell-intrinsic and -extrinsic mechanisms promote cell-type-specific cytokinetic diversity
Abstract
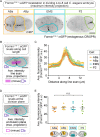
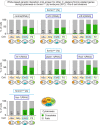
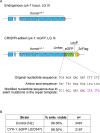
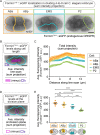
Cytokinesis, the physical division of one cell into two, is powered by constriction of an actomyosin contractile ring. It has long been assumed that all animal cells divide by a similar molecular mechanism, but growing evidence suggests that cytokinetic regulation in individual cell types has more variation than previously realized. In the four-cell Caenorhabditis elegans embryo, each blastomere has a distinct cell fate, specified by conserved pathways. Using fast-acting temperature-sensitive mutants and acute drug treatment, we identified cell-type-specific variation in the cytokinetic requirement for a robust forminCYK-1-dependent filamentous-actin (F-actin) cytoskeleton. In one cell (P2), this cytokinetic variation is cell-intrinsically regulated, whereas in another cell (EMS) this variation is cell-extrinsically regulated, dependent on both SrcSRC-1 signaling and direct contact with its neighbor cell, P2. Thus, both cell-intrinsic and -extrinsic mechanisms control cytokinetic variation in individual cell types and can protect against division failure when the contractile ring is weakened.
Keywords: C. elegans; Src kinase; actomyosin; cell biology; cell extrinsic; cell intrinsic; cytokinesis; developmental biology.
© 2018, Davies et al.
Conflict of interest statement
TD, HK, NR, BL, JD, MS, JC No competing interests declared
Figures


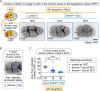
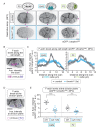

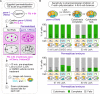
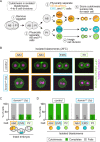
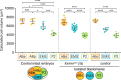
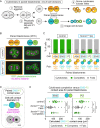
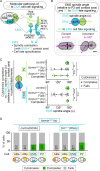
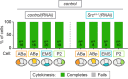
Comment in
-
Developmental Diversity in Cell Division Mechanisms.Dev Cell. 2018 Dec 3;47(5):535-536. doi: 10.1016/j.devcel.2018.11.028. Dev Cell. 2018. PMID: 30513295
Similar articles
-
The ARP2/3 complex prevents excessive formin activity during cytokinesis.Mol Biol Cell. 2019 Jan 1;30(1):96-107. doi: 10.1091/mbc.E18-07-0471. Epub 2018 Nov 7. Mol Biol Cell. 2019. PMID: 30403552 Free PMC article.
-
Germ fate determinants protect germ precursor cell division by reducing septin and anillin levels at the cell division plane.Mol Biol Cell. 2024 Jul 1;35(7):ar94. doi: 10.1091/mbc.E24-02-0096-T. Epub 2024 May 2. Mol Biol Cell. 2024. PMID: 38696255 Free PMC article.
-
A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans.Curr Biol. 2002 Dec 23;12(24):2066-75. doi: 10.1016/s0960-9822(02)01355-6. Curr Biol. 2002. PMID: 12498681
-
Cell cycle timing regulation during asynchronous divisions of the early C. elegans embryo.Exp Cell Res. 2015 Oct 1;337(2):243-8. doi: 10.1016/j.yexcr.2015.07.022. Epub 2015 Jul 23. Exp Cell Res. 2015. PMID: 26213213 Review.
-
C. elegans Embryonic Morphogenesis.Curr Top Dev Biol. 2016;116:597-616. doi: 10.1016/bs.ctdb.2015.11.012. Epub 2016 Feb 1. Curr Top Dev Biol. 2016. PMID: 26970644 Review.
Cited by
-
FHOD-1 is the only formin in Caenorhabditis elegans that promotes striated muscle growth and Z-line organization in a cell autonomous manner.Cytoskeleton (Hoboken). 2020 Oct;77(10):422-441. doi: 10.1002/cm.21639. Epub 2020 Nov 6. Cytoskeleton (Hoboken). 2020. PMID: 33103378 Free PMC article.
-
Complementary functions for the Ran gradient during division.Small GTPases. 2021 May;12(3):177-187. doi: 10.1080/21541248.2020.1725371. Epub 2020 Feb 14. Small GTPases. 2021. PMID: 32013678 Free PMC article. Review.
-
Profilin choreographs actin and microtubules in cells and cancer.Int Rev Cell Mol Biol. 2020;355:155-204. doi: 10.1016/bs.ircmb.2020.05.005. Epub 2020 Jul 16. Int Rev Cell Mol Biol. 2020. PMID: 32859370 Free PMC article.
-
A cooperative network at the nuclear envelope counteracts LINC-mediated forces during oogenesis in C. elegans.Sci Adv. 2023 Jul 14;9(28):eabn5709. doi: 10.1126/sciadv.abn5709. Epub 2023 Jul 12. Sci Adv. 2023. PMID: 37436986 Free PMC article.
-
Diversity is the spice of life: An overview of how cytokinesis regulation varies with cell type.Front Cell Dev Biol. 2022 Nov 7;10:1007614. doi: 10.3389/fcell.2022.1007614. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36420142 Free PMC article. Review.
References
-
- Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127:1887–1897. - PubMed
-
- Audhya A, Hyndman F, McLeod IX, Maddox AS, Yates JR, Desai A, Oegema K. A complex containing the sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. The Journal of Cell Biology. 2005;171:267–279. doi: 10.1083/jcb.200506124. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NIH R01GM105775/NH/NIH HHS/United States
- R01 GM117407/GM/NIGMS NIH HHS/United States
- NIH DP2OD008773/NH/NIH HHS/United States
- NIH R01GM117407/NH/NIH HHS/United States
- DP2 OD008773/OD/NIH HHS/United States
- R35 GM127049/GM/NIGMS NIH HHS/United States
- Charles H. Revson Senior Fellowship in Biomedical Science/Charles H. Revson Foundation/International
- FRM DEQ20160334869/Fondation pour la Recherche Médicale/International
- NIH R01AG045842/NH/NIH HHS/United States
- R01 AG045842/AG/NIA NIH HHS/United States
- ANR-16-CE13-0020-01/Agence Nationale de la Recherche/International
- R01 GM105775/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

