Synaptotagmin7 Is Overexpressed In Colorectal Cancer And Regulates Colorectal Cancer Cell Proliferation
- PMID: 30026831
- PMCID: PMC6036711
- DOI: 10.7150/jca.25098
Synaptotagmin7 Is Overexpressed In Colorectal Cancer And Regulates Colorectal Cancer Cell Proliferation
Abstract
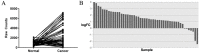
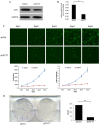
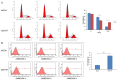
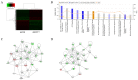
Purpose: Synaptotagmin7 (SYT7) belongs to the synaptotagmin gene family and plays an important role in synaptic transmission. However, the function of this gene in most human cancer especially in colorectal cancer (CRC) remains unknown. In this research, we examined SYT7's role in CRC and tried to reveal its underlying mechanism. Methods: We examined SYT7's expression levels in normal colorectal tissue and CRC tissues from 83 patients and analyzed the possible correlation between the expression level of SYT7 and pathological characteristics. The influences of SYT7 knockdown on cell growth were detected by Celigo image cytometer, colony formation assay, cell cycle analysis and apoptosis assay in vitro. The possible molecular mechanism was assessed using a microarray and Ingenuity Pathway Analysis. Results: Our results show that the expression of SYT7 is upregulated in colorectal cancer tissues in comparison with normal tissues and positively correlated with the pathological stage of colorectal cancer. (P=0.015). We examined SYT7's role in human colorectal cancer cell line RKO by using SYT7-shRNA and revealed that SYT7 knockdown inhibit cell proliferation (P=8.6E-5), clonogenic ability (P=4.5E-6) and promoted G2/M Phase arrest and apoptosis (P=4.6E-7). Multiple cancer-associated pathways regulated by SYT7 were unraveled by microarray and Ingenuity Pathway Analysis. Conclusions: Our study suggests that SYT7 plays an important role in the development of CRC and SYT7 may become a new therapeutic target in CRC.
Keywords: Synaptotagmin7; TCGA; apoptosis; colorectal cancer; proliferation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Silencing of synaptotagmin 7 regulates osteosarcoma cell proliferation, apoptosis, and migration.Histol Histopathol. 2020 Mar;35(3):303-312. doi: 10.14670/HH-18-174. Epub 2019 Oct 21. Histol Histopathol. 2020. PMID: 31631310
-
Downregulation of SYT7 inhibits glioblastoma growth by promoting cellular apoptosis.Mol Med Rep. 2017 Dec;16(6):9017-9022. doi: 10.3892/mmr.2017.7723. Epub 2017 Oct 4. Mol Med Rep. 2017. PMID: 28990113
-
SYT7 promotes breast cancer cells growth through the PI3K/AKT pathway.Transl Cancer Res. 2024 Jun 30;13(6):2767-2778. doi: 10.21037/tcr-24-7. Epub 2024 Jun 6. Transl Cancer Res. 2024. PMID: 38988943 Free PMC article.
-
SYT7 plays a role in promoting thyroid cancer by mediating HMGB3 ubiquitination.Endocr Relat Cancer. 2022 Mar 1;29(4):175-189. doi: 10.1530/ERC-21-0146. Endocr Relat Cancer. 2022. PMID: 35073278
-
SYT7 (synaptotagmin 7) promotes cervical squamous cell carcinoma.Heliyon. 2024 Jan 19;10(3):e24806. doi: 10.1016/j.heliyon.2024.e24806. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38314285 Free PMC article.
Cited by
-
Synaptotagmin 1 Suppresses Colorectal Cancer Metastasis by Inhibiting ERK/MAPK Signaling-Mediated Tumor Cell Pseudopodial Formation and Migration.Cancers (Basel). 2023 Nov 3;15(21):5282. doi: 10.3390/cancers15215282. Cancers (Basel). 2023. PMID: 37958455 Free PMC article.
-
SYT7 acts as an oncogene and a potential therapeutic target and was regulated by ΔNp63α in HNSCC.Cancer Cell Int. 2021 Dec 20;21(1):696. doi: 10.1186/s12935-021-02394-w. Cancer Cell Int. 2021. PMID: 34930262 Free PMC article.
-
Calcium Sensors in Neuronal Function and Dysfunction.Cold Spring Harb Perspect Biol. 2019 May 1;11(5):a035154. doi: 10.1101/cshperspect.a035154. Cold Spring Harb Perspect Biol. 2019. PMID: 30833454 Free PMC article. Review.
-
Silencing of synaptotagmin 7 regulates osteosarcoma cell proliferation, apoptosis, and migration.Histol Histopathol. 2020 Mar;35(3):303-312. doi: 10.14670/HH-18-174. Epub 2019 Oct 21. Histol Histopathol. 2020. PMID: 31631310
-
SYT12 plays a critical role in oral cancer and may be a novel therapeutic target.J Cancer. 2019 Aug 27;10(20):4913-4920. doi: 10.7150/jca.32582. eCollection 2019. J Cancer. 2019. PMID: 31598163 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

