Biodegradable and Bioactive PCL-PGS Core-Shell Fibers for Tissue Engineering
- PMID: 30023516
- PMCID: PMC6044571
- DOI: 10.1021/acsomega.7b00460
Biodegradable and Bioactive PCL-PGS Core-Shell Fibers for Tissue Engineering
Abstract
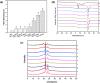
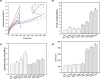
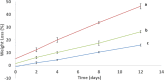
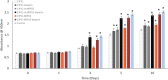
Poly(glycerol sebacate) (PGS) has increasingly become a desirable biomaterial due to its elastic mechanical properties, biodegradability, and biocompatibility. Here, we report microfibrous core-shell mats of polycaprolactone (PCL)-PGS prepared using wet-wet coaxial electrospinning. The anticoagulant heparin was immobilized onto the surface of these electrospun fiber mats, and they were evaluated for their chemical, mechanical, and biological properties. The core-shell structure of PCL-PGS provided tunable degradation and mechanical properties. The slowly degrading PCL provided structural integrity, and the fast degrading PGS component increased fiber elasticity. Young's modulus of PCL-PGS ranged from 5.6 to 15.7 MPa. The ultimate tensile stress ranged from 2.0 to 2.9 MPa, and these fibers showed elongation from 290 to 900%. The addition of PGS and grafting of heparin improved the attachment and proliferation of human umbilical vein endothelial cells. Core-shell PCL-PGS fibers demonstrate improved performance as three-dimensional fibrous mats for potential tissue-engineering applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering.Mater Sci Eng C Mater Biol Appl. 2020 Feb;107:110291. doi: 10.1016/j.msec.2019.110291. Epub 2019 Oct 8. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31761240 Free PMC article.
-
Design and characterization of dexamethasone-loaded poly (glycerol sebacate)-poly caprolactone/gelatin scaffold by coaxial electro spinning for soft tissue engineering.Mater Sci Eng C Mater Biol Appl. 2017 Sep 1;78:47-58. doi: 10.1016/j.msec.2017.04.047. Epub 2017 Apr 7. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28576011
-
Cardiogenic differentiation of mesenchymal stem cells on elastomeric poly (glycerol sebacate)/collagen core/shell fibers.World J Cardiol. 2013 Mar 26;5(3):28-41. doi: 10.4330/wjc.v5.i3.28. World J Cardiol. 2013. PMID: 23539543 Free PMC article.
-
Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications.Polymers (Basel). 2022 Jan 3;14(1):182. doi: 10.3390/polym14010182. Polymers (Basel). 2022. PMID: 35012203 Free PMC article. Review.
-
Recent trends in the application of protein electrospun fibers for loading food bioactive compounds.Food Chem X. 2023 Oct 6;20:100922. doi: 10.1016/j.fochx.2023.100922. eCollection 2023 Dec 30. Food Chem X. 2023. PMID: 38144745 Free PMC article. Review.
Cited by
-
Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering.Mater Sci Eng C Mater Biol Appl. 2020 Feb;107:110291. doi: 10.1016/j.msec.2019.110291. Epub 2019 Oct 8. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31761240 Free PMC article.
-
A Composite Microfiber for Biodegradable Stretchable Electronics.Micromachines (Basel). 2021 Aug 28;12(9):1036. doi: 10.3390/mi12091036. Micromachines (Basel). 2021. PMID: 34577680 Free PMC article.
-
Benefits of topical indigo naturalis nanofibrous patch on psoriatic skin: A transdermal strategy for botanicals.Mater Today Bio. 2023 Aug 2;22:100756. doi: 10.1016/j.mtbio.2023.100756. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37593218 Free PMC article.
-
Electrospun Fiber Mesh for High-Resolution Measurements of Oxygen Tension in Cranial Bone Defect Repair.ACS Appl Mater Interfaces. 2019 Sep 18;11(37):33548-33558. doi: 10.1021/acsami.9b08341. Epub 2019 Sep 4. ACS Appl Mater Interfaces. 2019. PMID: 31436082 Free PMC article.
-
Different methods of synthesizing poly(glycerol sebacate) (PGS): A review.Front Bioeng Biotechnol. 2022 Nov 30;10:1033827. doi: 10.3389/fbioe.2022.1033827. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36532580 Free PMC article. Review.
References
-
- Langer R.; Vacanti J. P. Tissue Engineering. Science 1993, 260, 920–926. - PubMed
-
- Nair L. S.; Laurencin C. T.. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. In Tissue Engineering I; Advances in Biochemical Engineering/Biotechnology, 2006; Vol. 102, pp 47–90. - PubMed
-
- Karande T.; Kaufmann J. J. M.; Agrawal C.. Function and Requirement of Synthetic Scaffolds in Tissue Engineering, 2nd ed.; CRC Press: Boca Raton, 2008.
-
- Igwe J.; Amini A.; Mikael P.; Laurencin C.; Nukavarapu S.. Nanostructured Scaffolds for Bone Tissue Engineering. In Active Implants and Scaffolds for Tissue Regeneration; Zilberman M., Ed.; Springer, 2011; Vol. 8, pp 169–192.
LinkOut - more resources
Full Text Sources
Other Literature Sources
