Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission
- PMID: 30022076
- PMCID: PMC6052000
- DOI: 10.1038/s41598-018-29060-y
Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission
Abstract
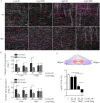
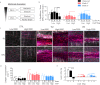
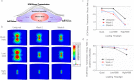
The extracellular matrix (ECM) is the primary biomechanical environment that interacts with tendon cells (tenocytes). Stresses applied via muscle contraction during skeletal movement transfer across structural hierarchies to the tenocyte nucleus in native uninjured tendons. Alterations to ECM structural and mechanical properties due to mechanical loading and tissue healing may affect this multiscale strain transfer and stress transmission through the ECM. This study explores the interface between dynamic loading and tendon healing across multiple length scales using living tendon explants. Results show that macroscale mechanical and structural properties are inferior following high magnitude dynamic loading (fatigue) in uninjured living tendon and that these effects propagate to the microscale. Although similar macroscale mechanical effects of dynamic loading are present in healing tendon compared to uninjured tendon, the microscale properties differed greatly during early healing. Regression analysis identified several variables (collagen and nuclear disorganization, cellularity, and F-actin) that directly predict nuclear deformation under loading. Finite element modeling predicted deficits in ECM stress transmission following fatigue loading and during healing. Together, this work identifies the multiscale response of tendon to dynamic loading and healing, and provides new insight into microenvironmental features that tenocytes may experience following injury and after cell delivery therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tendon healing affects the multiscale mechanical, structural and compositional response of tendon to quasi-static tensile loading.J R Soc Interface. 2018 Feb;15(139):20170880. doi: 10.1098/rsif.2017.0880. J R Soc Interface. 2018. PMID: 29467258 Free PMC article.
-
Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury.J Biomech. 2014 Jun 27;47(9):2028-34. doi: 10.1016/j.jbiomech.2013.10.054. Epub 2013 Nov 11. J Biomech. 2014. PMID: 24280564 Free PMC article.
-
Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading.Physiol Rev. 2004 Apr;84(2):649-98. doi: 10.1152/physrev.00031.2003. Physiol Rev. 2004. PMID: 15044685 Review.
-
In Vivo and In Vitro Mechanical Loading of Mouse Achilles Tendons and Tenocytes-A Pilot Study.Int J Mol Sci. 2020 Feb 15;21(4):1313. doi: 10.3390/ijms21041313. Int J Mol Sci. 2020. PMID: 32075290 Free PMC article.
-
The role of the tendon ECM in mechanotransduction: disruption and repair following overuse.Connect Tissue Res. 2022 Jan;63(1):28-42. doi: 10.1080/03008207.2021.1925663. Epub 2021 May 24. Connect Tissue Res. 2022. PMID: 34030531 Review.
Cited by
-
Collagen I: a kingpin for rotator cuff tendon pathology.Am J Transl Res. 2018 Nov 15;10(11):3291-3309. eCollection 2018. Am J Transl Res. 2018. PMID: 30662587 Free PMC article. Review.
-
Collagen denaturation in post-run Achilles tendons and Achilles tendinopathy: In vivo mechanophysiology and magnetic resonance imaging.Sci Adv. 2024 Oct 4;10(40):eado2015. doi: 10.1126/sciadv.ado2015. Epub 2024 Oct 2. Sci Adv. 2024. PMID: 39356750 Free PMC article.
-
Mechanics and differential healing outcomes of small and large defect injuries of the tendon-bone attachment in the rat rotator cuff.Connect Tissue Res. 2023 May;64(3):262-273. doi: 10.1080/03008207.2022.2152334. Epub 2022 Dec 16. Connect Tissue Res. 2023. PMID: 36524714 Free PMC article.
-
Tendon Biomechanics and Crimp Properties Following Fatigue Loading Are Influenced by Tendon Type and Age in Mice.J Orthop Res. 2020 Jan;38(1):36-42. doi: 10.1002/jor.24407. Epub 2019 Jul 23. J Orthop Res. 2020. PMID: 31286548 Free PMC article.
-
In Vitro Cellular Strain Models of Tendon Biology and Tenogenic Differentiation.Front Bioeng Biotechnol. 2022 Feb 15;10:826748. doi: 10.3389/fbioe.2022.826748. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35242750 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

