Middle East Respiratory Syndrome Coronavirus Spike Protein Is Not Activated Directly by Cellular Furin during Viral Entry into Target Cells
- PMID: 30021905
- PMCID: PMC6146822
- DOI: 10.1128/JVI.00683-18
Middle East Respiratory Syndrome Coronavirus Spike Protein Is Not Activated Directly by Cellular Furin during Viral Entry into Target Cells
Abstract
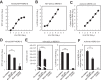
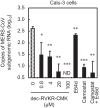
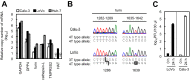
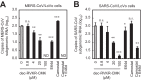
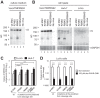
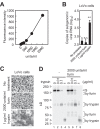
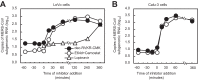
Middle East respiratory syndrome coronavirus (MERS-CoV) utilizes host cellular proteases to enter cells. A previous report shows that furin, which is distributed mainly in the Golgi apparatus and cycled to the cell surface and endosomes, proteolytically activates the MERS-CoV spike (S) protein following receptor binding to mediate fusion between the viral and cellular membranes. In this study, we reexamined furin usage by MERS-CoV using a real-time PCR-based virus cell entry assay after inhibition of cellular proteases. We found that the furin inhibitor dec-RVKR-CMK blocked entry of MERS-CoV harboring an S protein lacking furin cleavage sites; it even blocked entry into furin-deficient LoVo cells. In addition, dec-RVKR-CMK inhibited not only the enzymatic activity of furin but also those of cathepsin L, cathepsin B, trypsin, papain, and TMPRSS2. Furthermore, a virus cell entry assay and a cell-cell fusion assay provided no evidence that the S protein was activated by exogenous furin. Therefore, we conclude that furin does not play a role in entry of MERS-CoV into cells and that the inhibitory effect of dec-RVKR-CMK is specific for TMPRSS2 and cathepsin L rather than furin.IMPORTANCE Previous studies using the furin inhibitor dec-RVKR-CMK suggest that MERS-CoV utilizes a cellular protease, furin, to activate viral glycoproteins during cell entry. However, we found that dec-RVKR-CMK inhibits not only furin but also other proteases. Furthermore, we found no evidence that MERS-CoV uses furin. These findings suggest that previous studies in the virology field based on dec-RVKR-CMK should be reexamined carefully. Here we describe appropriate experiments that can be used to assess the effect of protease inhibitors on virus cell entry.
Keywords: TMPRSS2; cathepsin L; coronavirus; dec-RVKR-CMK; furin.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein.Sci Rep. 2018 Nov 9;8(1):16597. doi: 10.1038/s41598-018-34859-w. Sci Rep. 2018. PMID: 30413791 Free PMC article.
-
Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay.Antimicrob Agents Chemother. 2016 Oct 21;60(11):6532-6539. doi: 10.1128/AAC.01043-16. Print 2016 Nov. Antimicrob Agents Chemother. 2016. PMID: 27550352 Free PMC article.
-
Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry.J Virol. 2016 Dec 16;91(1):e01387-16. doi: 10.1128/JVI.01387-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27733646 Free PMC article.
-
[Development of peptidic MERS-CoV entry inhibitors].Yao Xue Xue Bao. 2015 Dec;50(12):1513-9. Yao Xue Xue Bao. 2015. PMID: 27169270 Review. Chinese.
-
Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis.Virus Res. 2015 Apr 16;202:120-34. doi: 10.1016/j.virusres.2014.11.021. Epub 2014 Nov 22. Virus Res. 2015. PMID: 25445340 Free PMC article. Review.
Cited by
-
COVID-19 signalome: Potential therapeutic interventions.Cell Signal. 2023 Mar;103:110559. doi: 10.1016/j.cellsig.2022.110559. Epub 2022 Dec 13. Cell Signal. 2023. PMID: 36521656 Free PMC article. Review.
-
Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein.Sci Rep. 2018 Nov 9;8(1):16597. doi: 10.1038/s41598-018-34859-w. Sci Rep. 2018. PMID: 30413791 Free PMC article.
-
A Review on Expression, Pathological Roles, and Inhibition of TMPRSS2, the Serine Protease Responsible for SARS-CoV-2 Spike Protein Activation.Scientifica (Cairo). 2021 Jul 24;2021:2706789. doi: 10.1155/2021/2706789. eCollection 2021. Scientifica (Cairo). 2021. PMID: 34336361 Free PMC article. Review.
-
In Vitro Models for Studying Entry, Tissue Tropism, and Therapeutic Approaches of Highly Pathogenic Coronaviruses.Biomed Res Int. 2021 Jun 21;2021:8856018. doi: 10.1155/2021/8856018. eCollection 2021. Biomed Res Int. 2021. PMID: 34239932 Free PMC article. Review.
-
The Physiological TMPRSS2 Inhibitor HAI-2 Alleviates SARS-CoV-2 Infection.J Virol. 2021 May 24;95(12):e00434-21. doi: 10.1128/JVI.00434-21. Print 2021 May 24. J Virol. 2021. PMID: 33789993 Free PMC article. No abstract available.
References
-
- Gierer S, Bertram S, Kaup F, Wrensch F, Heurich A, Krämer-Kühl A, Welsch K, Winkler M, Meyer B, Drosten C, Dittmer U, von Hahn T, Simmons G, Hofmann H, Pöhlmann S. 2013. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol 87:5502–5511. doi:10.1128/JVI.00128-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

