Luciferase complementation based-detection of G-protein-coupled receptor activity
- PMID: 30014734
- PMCID: PMC7365683
- DOI: 10.2144/btn-2018-0039
Luciferase complementation based-detection of G-protein-coupled receptor activity
Abstract
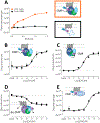
Protein complementation assays (PCA) are used as pharmacological tools, enabling a wide array of applications, ranging from studies of protein-protein interactions to second messenger effects. Methods to detect activities of G protein-coupled receptors (GPCRs) have particular relevance for drug screening. Recent development of an engineered luciferase NanoLuc created the possibility of generating a novel PCA, which in turn could open a new avenue for developing drug screening assays. Here we identified a novel split position for NanoLuc and demonstrated its use in a series of fusion constructs to detect the activity of GPCRs. The split construct can be applied to a variety of pharmacological screening systems.
Keywords: GPCR; assay development; biosensor; complementation; dopamine receptor; luciferase.
Figures
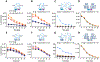
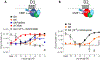
Similar articles
-
Split luciferase complementation for analysis of intracellular signaling.Anal Sci. 2014;30(5):539-44. doi: 10.2116/analsci.30.539. Anal Sci. 2014. PMID: 24813951 Review.
-
Rapid and high-sensitivity cell-based assays of protein-protein interactions using split click beetle luciferase complementation: an approach to the study of G-protein-coupled receptors.Anal Chem. 2010 Mar 15;82(6):2552-60. doi: 10.1021/ac100104q. Anal Chem. 2010. PMID: 20180537
-
Luciferase Complementation Approaches to Measure GPCR Signaling Kinetics and Bias.Methods Mol Biol. 2021;2268:249-274. doi: 10.1007/978-1-0716-1221-7_17. Methods Mol Biol. 2021. PMID: 34085274
-
Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells.J Biol Chem. 2018 May 11;293(19):7466-7473. doi: 10.1074/jbc.RA118.001975. Epub 2018 Mar 9. J Biol Chem. 2018. PMID: 29523687 Free PMC article.
-
Luminescence- and Fluorescence-Based Complementation Assays to Screen for GPCR Oligomerization: Current State of the Art.Int J Mol Sci. 2019 Jun 17;20(12):2958. doi: 10.3390/ijms20122958. Int J Mol Sci. 2019. PMID: 31213021 Free PMC article. Review.
Cited by
-
A general method for quantifying ligand binding to unmodified receptors using Gaussia luciferase.J Biol Chem. 2021 Jan-Jun;296:100366. doi: 10.1016/j.jbc.2021.100366. Epub 2021 Feb 2. J Biol Chem. 2021. PMID: 33545176 Free PMC article.
-
Development and characterization of new tools for detecting poly(ADP-ribose) in vitro and in vivo.Elife. 2022 Apr 27;11:e72464. doi: 10.7554/eLife.72464. Elife. 2022. PMID: 35476036 Free PMC article.
-
A novel luminescence-based β-arrestin recruitment assay for unmodified receptors.J Biol Chem. 2021 Jan-Jun;296:100503. doi: 10.1016/j.jbc.2021.100503. Epub 2021 Mar 5. J Biol Chem. 2021. PMID: 33684444 Free PMC article.
-
Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors.Annu Rev Anal Chem (Palo Alto Calif). 2019 Jun 12;12(1):129-150. doi: 10.1146/annurev-anchem-061318-115027. Epub 2019 Feb 20. Annu Rev Anal Chem (Palo Alto Calif). 2019. PMID: 30786216 Free PMC article. Review.
-
A dynamic and screening-compatible nanoluciferase-based complementation assay enables profiling of individual GPCR-G protein interactions.J Biol Chem. 2019 Mar 15;294(11):4079-4090. doi: 10.1074/jbc.RA118.006231. Epub 2018 Dec 28. J Biol Chem. 2019. PMID: 30593506 Free PMC article.
References
-
- Azad T, Tashakor A, and Hosseinkhani S. 2014. Split-luciferase complementary assay: applications, recent developments, and future perspectives. Analytical and Bioanalytical Chemistry 406:5541–5560. - PubMed
-
- Remy I and Michnick SW. 2007. Application of protein-fragment complementation assays in cell biology. Biotechniques 42:137–145. - PubMed
-
- Morell M, Ventura S, and Aviles FX. 2009. Protein complementation assays: approaches for the in vivo analysis of protein interactions. FEBS Lett 583:1684–1691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
