A non-canonical GTPase interaction enables ORP1L-Rab7-RILP complex formation and late endosome positioning
- PMID: 30012887
- PMCID: PMC6130934
- DOI: 10.1074/jbc.RA118.001854
A non-canonical GTPase interaction enables ORP1L-Rab7-RILP complex formation and late endosome positioning
Abstract
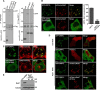
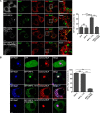
Endosomal transport represents the primary mode for intracellular trafficking and signaling transduction and thus has to be tightly controlled. The molecular processes controlling the endosomal positioning utilize several large protein complexes, one of which contains the small GTPase Rab7, Rab-interacting lysosomal protein (RILP), and oxysterol-binding protein-related protein 1 (ORP1L). Rab7 is known to interact with RILP through a canonical binding site termed the effector-interacting switch region, but it is not clear how Rab7 interacts with ORP1L, limiting our understanding of the overall process. Here, we report structural and biochemical investigation of the Rab7-ORP1L interaction. We found that, contrary to prior studies, the interaction between Rab7 and the N-terminal ankyrin repeat domain (ARDN) of ORP1L is independent of Rab7's GTP- or GDP-binding state. Moreover, we show that Rab7 interacts with ORP1L ARDN via a unique region consisting of helix3 (α3) and 310-helix 2 (η2). This architecture leaves the canonical effector-interacting switch regions available for RILP binding and thus allows formation of the ORP1L-Rab7-RILP tripartite complex. Mutational disruption of the interacting interface between ORP1L and Rab7 compromised the ability of ORP1L-Rab7-RILP to regulate the late endosome positioning. Collectively, our results again manifested the versatility in the interaction between GTPase and its effector.
Keywords: GTPase; ORP1L; RILP; Rab7; cholesterol; crystal structure; endosome; protein complex; protein-protein interaction; trafficking.
© 2018 Ma et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin.J Cell Biol. 2007 Feb 12;176(4):459-71. doi: 10.1083/jcb.200606077. Epub 2007 Feb 5. J Cell Biol. 2007. PMID: 17283181 Free PMC article.
-
Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L.J Cell Sci. 2013 Aug 1;126(Pt 15):3462-74. doi: 10.1242/jcs.129270. Epub 2013 May 31. J Cell Sci. 2013. PMID: 23729732
-
Structural basis of human ORP1-Rab7 interaction for the late-endosome and lysosome targeting.PLoS One. 2019 Feb 5;14(2):e0211724. doi: 10.1371/journal.pone.0211724. eCollection 2019. PLoS One. 2019. PMID: 30721249 Free PMC article.
-
Rab7: role of its protein interaction cascades in endo-lysosomal traffic.Cell Signal. 2011 Mar;23(3):516-21. doi: 10.1016/j.cellsig.2010.09.012. Epub 2010 Sep 21. Cell Signal. 2011. PMID: 20851765 Review.
-
This Is the End: Regulation of Rab7 Nucleotide Binding in Endolysosomal Trafficking and Autophagy.Front Cell Dev Biol. 2018 Oct 2;6:129. doi: 10.3389/fcell.2018.00129. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30333976 Free PMC article. Review.
Cited by
-
PDZD8 mediates a Rab7-dependent interaction of the ER with late endosomes and lysosomes.Proc Natl Acad Sci U S A. 2019 Nov 5;116(45):22619-22623. doi: 10.1073/pnas.1913509116. Epub 2019 Oct 21. Proc Natl Acad Sci U S A. 2019. PMID: 31636202 Free PMC article.
-
Regulation of Endosomal Trafficking by Rab7 and Its Effectors in Neurons: Clues from Charcot-Marie-Tooth 2B Disease.Biomolecules. 2023 Sep 16;13(9):1399. doi: 10.3390/biom13091399. Biomolecules. 2023. PMID: 37759799 Free PMC article. Review.
-
VP28 interacts with PmRab7 irrespective of its nucleotide state.Sci Rep. 2024 Nov 13;14(1):27803. doi: 10.1038/s41598-024-79310-5. Sci Rep. 2024. PMID: 39537865 Free PMC article.
-
Choreographing the motor-driven endosomal dance.J Cell Sci. 2023 Mar 1;136(5):jcs259689. doi: 10.1242/jcs.259689. Epub 2022 Nov 16. J Cell Sci. 2023. PMID: 36382597 Free PMC article. Review.
-
Formation and Maturation of the Phagosome: A Key Mechanism in Innate Immunity against Intracellular Bacterial Infection.Microorganisms. 2020 Aug 25;8(9):1298. doi: 10.3390/microorganisms8091298. Microorganisms. 2020. PMID: 32854338 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

