Bioactive hydrogels for bone regeneration
- PMID: 30003179
- PMCID: PMC6038268
- DOI: 10.1016/j.bioactmat.2018.05.006
Bioactive hydrogels for bone regeneration
Abstract
Bone self-healing is limited and generally requires external intervention to augment bone repair and regeneration. While traditional methods for repairing bone defects such as autografts, allografts, and xenografts have been widely used, they all have corresponding disadvantages, thus limiting their clinical use. Despite the development of a variety of biomaterials, including metal implants, calcium phosphate cements (CPC), hydroxyapatite, etc., the desired therapeutic effect is not fully achieved. Currently, polymeric scaffolds, particularly hydrogels, are of interest and their unique configurations and tunable physicochemical properties have been extensively studied. This review will focus on the applications of various cutting-edge bioactive hydrogels systems in bone regeneration, as well as their advantages and limitations. We will examine the composition and defects of the bone, discuss the current biomaterials for bone regeneration, and classify recently developed polymeric materials for hydrogel synthesis. We will also elaborate on the properties of desirable hydrogels as well as the fabrication techniques and different delivery strategies. Finally, the existing challenges, considerations, and the future prospective of hydrogels in bone regeneration will be outlined.
Keywords: Biomaterials; Bone regeneration; Hydrogel; Tissue engineering.
Figures


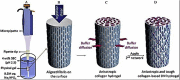
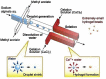

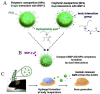
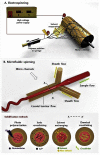
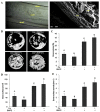

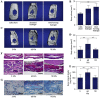
Similar articles
-
Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration.Acta Biomater. 2021 Dec;136:1-36. doi: 10.1016/j.actbio.2021.09.034. Epub 2021 Sep 23. Acta Biomater. 2021. PMID: 34562661 Review.
-
Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration.Acta Biomater. 2020 Aug;112:262-273. doi: 10.1016/j.actbio.2020.05.034. Epub 2020 Jun 1. Acta Biomater. 2020. PMID: 32497742 Free PMC article.
-
Three-dimensional bioactive hydrogel-based scaffolds for bone regeneration in implant dentistry.Mater Sci Eng C Mater Biol Appl. 2021 May;124:112055. doi: 10.1016/j.msec.2021.112055. Epub 2021 Mar 24. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33947549 Review.
-
Self-healing hydrogels for bone defect repair.RSC Adv. 2023 Jun 5;13(25):16773-16788. doi: 10.1039/d3ra01700a. eCollection 2023 Jun 5. RSC Adv. 2023. PMID: 37283866 Free PMC article. Review.
-
Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications.Acta Biomater. 2017 Oct 15;62:42-63. doi: 10.1016/j.actbio.2017.07.028. Epub 2017 Jul 20. Acta Biomater. 2017. PMID: 28736220 Review.
Cited by
-
Biomechanical Stimulation of Muscle Constructs Influences Phenotype of Bone Constructs by Modulating Myokine Secretion.JBMR Plus. 2023 Aug 15;7(11):e10804. doi: 10.1002/jbm4.10804. eCollection 2023 Nov. JBMR Plus. 2023. PMID: 38025033 Free PMC article.
-
Biomimetic Mineralization of Tannic Acid-Supplemented HEMA/SBMA Nanocomposite Hydrogels.Polymers (Basel). 2021 May 22;13(11):1697. doi: 10.3390/polym13111697. Polymers (Basel). 2021. PMID: 34067423 Free PMC article.
-
Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review.Gels. 2023 Nov 8;9(11):885. doi: 10.3390/gels9110885. Gels. 2023. PMID: 37998975 Free PMC article. Review.
-
Methacrylated Silk Fibroin Additive Manufacturing of Shape Memory Constructs with Possible Application in Bone Regeneration.Gels. 2022 Dec 16;8(12):833. doi: 10.3390/gels8120833. Gels. 2022. PMID: 36547356 Free PMC article.
-
Incorporation of Natural and Recombinant Collagen Proteins within Fmoc-Based Self-Assembling Peptide Hydrogels.Gels. 2022 Apr 21;8(5):254. doi: 10.3390/gels8050254. Gels. 2022. PMID: 35621553 Free PMC article.
References
-
- Simeonov M.S., Apostolov A.A., Vassileva E.D. In situ calcium phosphate deposition in hydrogels of poly(acrylic acid)-polyacrylamide interpenetrating polymer networks. RSC Adv. 2016;6:16274–16284.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

