Interaction Between Macrophage Migration Inhibitory Factor and CD74 in Human Immunodeficiency Virus Type I Infected Primary Monocyte-Derived Macrophages Triggers the Production of Proinflammatory Mediators and Enhances Infection of Unactivated CD4+ T Cells
- PMID: 29997630
- PMCID: PMC6030361
- DOI: 10.3389/fimmu.2018.01494
Interaction Between Macrophage Migration Inhibitory Factor and CD74 in Human Immunodeficiency Virus Type I Infected Primary Monocyte-Derived Macrophages Triggers the Production of Proinflammatory Mediators and Enhances Infection of Unactivated CD4+ T Cells
Abstract
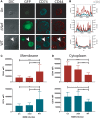
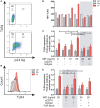
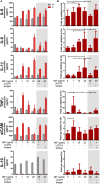
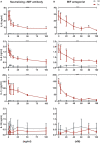
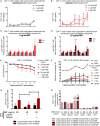
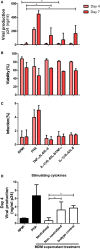
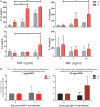
Understanding the mechanisms of human immunodeficiency virus type I (HIV-1) pathogenesis would facilitate the identification of new therapeutic targets to control the infection in face of current antiretroviral therapy limitations. CD74 membrane expression is upregulated in HIV-1-infected cells and the magnitude of its modulation correlates with immune hyperactivation in HIV-infected individuals. In addition, plasma level of the CD74 activating ligand macrophage migration inhibitory factor (MIF) is increased in infected subjects. However, the role played by MIF/CD74 interaction in HIV pathogenesis remains unexplored. Here, we studied the effect of MIF/CD74 interaction on primary HIV-infected monocyte-derived macrophages (MDMs) and its implications for HIV immunopathogenesis. Confocal immunofluorescence analysis of CD74 and CD44 (the MIF signal transduction co-receptor) expression indicated that both molecules colocalized at the plasma membrane specifically in wild-type HIV-infected MDMs. Treatment of infected MDMs with MIF resulted in an MIF-dependent increase in TLR4 expression. Similarly, there was a dose-dependent increase in the production of IL-6, IL-8, TNFα, IL-1β, and sICAM compared to the no-MIF condition, specifically from infected MDMs. Importantly, the effect observed on IL-6, IL-8, TNFα, and IL-1β was abrogated by impeding MIF interaction with CD74. Moreover, the use of a neutralizing αMIF antibody or an MIF antagonist reverted these effects, supporting the specificity of the results. Treatment of unactivated CD4+ T-cells with MIF-treated HIV-infected MDM-derived culture supernatants led to enhanced permissiveness to HIV-1 infection. This effect was lost when CD4+ T-cells were treated with supernatants derived from infected MDMs in which CD74/MIF interaction had been blocked. Moreover, the enhanced permissiveness of unactivated CD4+ T-cells was recapitulated by exogenous addition of IL-6, IL-8, IL-1β, and TNFα, or abrogated by neutralizing its biological activity using specific antibodies. Results obtained with BAL and NL4-3 HIV laboratory strains were reproduced using transmitted/founder primary isolates. This evidence indicated that MIF/CD74 interaction resulted in a higher production of proinflammatory cytokines from HIV-infected MDMs. This caused the generation of an inflammatory microenvironment which predisposed unactivated CD4+ T-cells to HIV-1 infection, which might contribute to viral spreading and reservoir seeding. Overall, these results support a novel role of the MIF/CD74 axis in HIV pathogenesis that deserves further investigation.
Keywords: CD4+ T-cells; CD74; human immunodeficiency virus; immunopathogenesis; macrophage migration inhibitory factor; primary monocyte-derived macrophages.
Figures

Similar articles
-
Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection.Viruses. 2022 Oct 9;14(10):2218. doi: 10.3390/v14102218. Viruses. 2022. PMID: 36298774 Free PMC article.
-
Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells.J Neuroinflammation. 2012 Jun 22;9:138. doi: 10.1186/1742-2094-9-138. J Neuroinflammation. 2012. PMID: 22727020 Free PMC article.
-
Proinflammatory Signature of the Dysfunctional Endothelium in Pulmonary Hypertension. Role of the Macrophage Migration Inhibitory Factor/CD74 Complex.Am J Respir Crit Care Med. 2015 Oct 15;192(8):983-97. doi: 10.1164/rccm.201402-0322OC. Am J Respir Crit Care Med. 2015. PMID: 26203495
-
[Deep lung--cellular reaction to HIV].Rev Port Pneumol. 2007 Mar-Apr;13(2):175-212. Rev Port Pneumol. 2007. PMID: 17492233 Review. Portuguese.
-
Cytokines and HIV-1: interactions and clinical implications.Antivir Chem Chemother. 2001 May;12(3):133-50. doi: 10.1177/095632020101200301. Antivir Chem Chemother. 2001. PMID: 12959322 Review.
Cited by
-
The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective.Biomedicines. 2023 Dec 19;12(1):2. doi: 10.3390/biomedicines12010002. Biomedicines. 2023. PMID: 38275363 Free PMC article. Review.
-
Landscape of Exhausted T Cells in Tuberculosis Revealed by Single-Cell Sequencing.Microbiol Spectr. 2023 Mar 14;11(2):e0283922. doi: 10.1128/spectrum.02839-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36916943 Free PMC article.
-
Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection.Viruses. 2022 Oct 9;14(10):2218. doi: 10.3390/v14102218. Viruses. 2022. PMID: 36298774 Free PMC article.
-
Altered immune cell in human severe acute pancreatitis revealed by single-cell RNA sequencing.Front Immunol. 2024 Sep 20;15:1354926. doi: 10.3389/fimmu.2024.1354926. eCollection 2024. Front Immunol. 2024. PMID: 39372399 Free PMC article.
-
The NeuroinflammatoryPotential of HIV-1 NefVariants in Modulating the Gene Expression Profile of Astrocytes.Cells. 2022 Oct 17;11(20):3256. doi: 10.3390/cells11203256. Cells. 2022. PMID: 36291123 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

