Prespliceosome structure provides insights into spliceosome assembly and regulation
- PMID: 29995849
- PMCID: PMC6141012
- DOI: 10.1038/s41586-018-0323-8
Prespliceosome structure provides insights into spliceosome assembly and regulation
Abstract
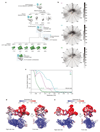
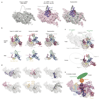
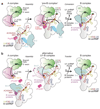
The spliceosome catalyses the excision of introns from pre-mRNA in two steps, branching and exon ligation, and is assembled from five small nuclear ribonucleoprotein particles (snRNPs; U1, U2, U4, U5, U6) and numerous non-snRNP factors1. For branching, the intron 5' splice site and the branch point sequence are selected and brought by the U1 and U2 snRNPs into the prespliceosome1, which is a focal point for regulation by alternative splicing factors2. The U4/U6.U5 tri-snRNP subsequently joins the prespliceosome to form the complete pre-catalytic spliceosome. Recent studies have revealed the structural basis of the branching and exon-ligation reactions3, however, the structural basis of the early events in spliceosome assembly remains poorly understood4. Here we report the cryo-electron microscopy structure of the yeast Saccharomyces cerevisiae prespliceosome at near-atomic resolution. The structure reveals an induced stabilization of the 5' splice site in the U1 snRNP, and provides structural insights into the functions of the human alternative splicing factors LUC7-like (yeast Luc7) and TIA-1 (yeast Nam8), both of which have been linked to human disease5,6. In the prespliceosome, the U1 snRNP associates with the U2 snRNP through a stable contact with the U2 3' domain and a transient yeast-specific contact with the U2 SF3b-containing 5' region, leaving its tri-snRNP-binding interface fully exposed. The results suggest mechanisms for 5' splice site transfer to the U6 ACAGAGA region within the assembled spliceosome and for its subsequent conversion to the activation-competent B-complex spliceosome7,8. Taken together, the data provide a working model to investigate the early steps of spliceosome assembly.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








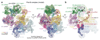
Similar articles
-
Structure of a pre-catalytic spliceosome.Nature. 2017 Jun 29;546(7660):617-621. doi: 10.1038/nature22799. Epub 2017 May 22. Nature. 2017. PMID: 28530653 Free PMC article.
-
Mechanism of 5' splice site transfer for human spliceosome activation.Science. 2019 Apr 26;364(6438):362-367. doi: 10.1126/science.aax3289. Epub 2019 Apr 11. Science. 2019. PMID: 30975767 Free PMC article.
-
Structure of a spliceosome remodelled for exon ligation.Nature. 2017 Feb 16;542(7641):377-380. doi: 10.1038/nature21078. Epub 2017 Jan 11. Nature. 2017. PMID: 28076345 Free PMC article.
-
CryoEM structures of two spliceosomal complexes: starter and dessert at the spliceosome feast.Curr Opin Struct Biol. 2016 Feb;36:48-57. doi: 10.1016/j.sbi.2015.12.005. Epub 2016 Jan 21. Curr Opin Struct Biol. 2016. PMID: 26803803 Free PMC article. Review.
-
RNA Splicing by the Spliceosome.Annu Rev Biochem. 2020 Jun 20;89:359-388. doi: 10.1146/annurev-biochem-091719-064225. Epub 2019 Dec 3. Annu Rev Biochem. 2020. PMID: 31794245 Review.
Cited by
-
Branch site recognition by the spliceosome.RNA. 2024 Oct 16;30(11):1397-1407. doi: 10.1261/rna.080198.124. RNA. 2024. PMID: 39187383 Free PMC article. Review.
-
Synergistic roles for human U1 snRNA stem-loops in pre-mRNA splicing.RNA Biol. 2021 Dec;18(12):2576-2593. doi: 10.1080/15476286.2021.1932360. Epub 2021 Jun 9. RNA Biol. 2021. PMID: 34105434 Free PMC article.
-
Zinc finger structure determination by NMR: Why zinc fingers can be a handful.Prog Nucl Magn Reson Spectrosc. 2022 Jun-Aug;130-131:62-105. doi: 10.1016/j.pnmrs.2022.07.001. Epub 2022 Jul 15. Prog Nucl Magn Reson Spectrosc. 2022. PMID: 36113918 Free PMC article. Review.
-
Alternative Splicing in Human Biology and Disease.Methods Mol Biol. 2022;2537:1-19. doi: 10.1007/978-1-0716-2521-7_1. Methods Mol Biol. 2022. PMID: 35895255
-
Proteomics Identifies LUC7L3 as a Prognostic Biomarker for Hepatocellular Carcinoma.Curr Issues Mol Biol. 2024 Apr 27;46(5):4004-4020. doi: 10.3390/cimb46050247. Curr Issues Mol Biol. 2024. PMID: 38785515 Free PMC article.
References
-
- Papasaikas P, Valcarcel J. The spliceosome: The ultimate RNA chaperone and sculptor. Trends Biochem Sci. 2016;41:33–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

