The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target
- PMID: 29989029
- PMCID: PMC6034748
- DOI: 10.18632/oncotarget.25615
The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target
Abstract
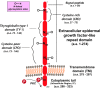
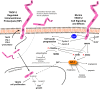
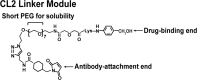
TROP-2 is a glycoprotein first described as a surface marker of trophoblast cells, but subsequently shown to be increased in many solid cancers, with lower expression in certain normal tissues. It regulates cancer growth, invasion and spread by several signaling pathways, and has a role in stem cell biology and other diseases. This review summarizes TROP-2's properties, especially in cancer, and particularly its role as a target for antibody-drug conjugates (ADC) or immunotherapy. When the irinotecan metabolite, SN-38, is conjugated to a humanized anti-TROP-2 antibody (sacituzumab govitecan), it shows potent broad anticancer activity in human cancer xenografts and in patients with advanced triple-negative breast, non-small cell and small-cell lung, as well as urothelial cancers.
Keywords: TACSTD2; TROP-2; antibody-drug conjugates; immunotherapy; sacituzumab govitecan.
Conflict of interest statement
CONFLICTS OF INTEREST Drs. Goldenberg and Sharkey own Immunomedics stock or stock options, and Dr. Goldenberg holds patented inventions. Dr. Goldenberg is the founder of the Center for Molecular Medicine and Immunology (CMMI), and also the retired founder of Immunomedics, Inc., and IBC Pharmaceuticals, Inc.
Figures

Similar articles
-
Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan.MAbs. 2019 Aug/Sep;11(6):987-995. doi: 10.1080/19420862.2019.1632115. Epub 2019 Jul 18. MAbs. 2019. PMID: 31208270 Free PMC article. Review.
-
Cervical carcinomas that overexpress human trophoblast cell-surface marker (Trop-2) are highly sensitive to the antibody-drug conjugate sacituzumab govitecan.Sci Rep. 2020 Jan 22;10(1):973. doi: 10.1038/s41598-020-58009-3. Sci Rep. 2020. PMID: 31969666 Free PMC article.
-
Preclinical Activity of Sacituzumab Govitecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2 (Trop-2) Linked to the Active Metabolite of Irinotecan (SN-38), in Ovarian Cancer.Front Oncol. 2020 Feb 12;10:118. doi: 10.3389/fonc.2020.00118. eCollection 2020. Front Oncol. 2020. PMID: 32117765 Free PMC article.
-
Trop-2 protein as a therapeutic target: A focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers.Bosn J Basic Med Sci. 2022 Feb 1;22(1):14-21. doi: 10.17305/bjbms.2021.6100. Bosn J Basic Med Sci. 2022. PMID: 34181512 Free PMC article. Review.
-
Sacituzumab govitecan for hormone receptor-positive and triple-negative breast cancers.Mol Cell Pharmacol. 2023;15(1):1-5. Mol Cell Pharmacol. 2023. PMID: 37090944 Free PMC article.
Cited by
-
An overview of antibody-drug conjugates in oncological practice.Ther Adv Med Oncol. 2020 Oct 4;12:1758835920962997. doi: 10.1177/1758835920962997. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 33088347 Free PMC article. Review.
-
Sacituzumab govitecan in HR+HER2- metastatic breast cancer: the randomized phase 3 EVER-132-002 trial.Nat Med. 2024 Dec;30(12):3709-3716. doi: 10.1038/s41591-024-03269-z. Epub 2024 Oct 1. Nat Med. 2024. PMID: 39354196 Free PMC article. Clinical Trial.
-
A literature review of the promising future of TROP2: a potential drug therapy target.Ann Transl Med. 2022 Dec;10(24):1403. doi: 10.21037/atm-22-5976. Ann Transl Med. 2022. PMID: 36660684 Free PMC article. Review.
-
Antibody-Drug Conjugates in Uro-Oncology.Target Oncol. 2022 May;17(3):203-221. doi: 10.1007/s11523-022-00872-3. Epub 2022 May 14. Target Oncol. 2022. PMID: 35567672 Review.
-
Triple negative breast cancer: approved treatment options and their mechanisms of action.J Cancer Res Clin Oncol. 2023 Jul;149(7):3701-3719. doi: 10.1007/s00432-022-04189-6. Epub 2022 Aug 17. J Cancer Res Clin Oncol. 2023. PMID: 35976445 Free PMC article.
References
-
- Jackson SE, Chester JD. Personalised cancer medicine. Int J Cancer. 2015;137:262–266. - PubMed
-
- Griffin J. The biology of signal transduction inhibition: basic science to novel therapies. Semin Oncol. 2001;28:3–8. - PubMed
-
- Virji MA, Mercer DW, Herberman RB. Tumor markers in cancer diagnosis and prognosis. CA Cancer J Clin. 1988;38:104–126. - PubMed
-
- Sharkey RM, Goldenberg DM. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J Clin. 2006;56:226–243. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

