Perforated jejunitis in a child with acute lymphoblastic leukemia treated with pegaspargase
- PMID: 29988797
- PMCID: PMC6030564
- DOI: 10.1016/j.radcr.2018.02.017
Perforated jejunitis in a child with acute lymphoblastic leukemia treated with pegaspargase
Abstract
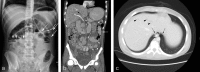
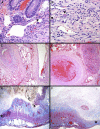
Survival rates of children with acute lymphoblastic leukemia have improved since the incorporation of asparaginase in the treatment protocol, but the medication has potential serious complications, including vascular thrombosis. Here, we describe the case of a 13-year-old boy with pre-T-cell acute lymphoblastic leukemia whose treatment course was complicated by perforated jejunitis requiring resection of a portion of his small bowel. Pathologic assessment showed transmural ischemia, mesenteric venous and arterial thrombi, and scattered cytomegalovirus inclusion bodies. Pediatric mesenteric ischemia is rare, and its consideration in patients treated with asparaginase is discussed.
Keywords: Acute lymphoblastic leukemia; Acute mesenteric ischemia; Asparaginase; Complications; Computed tomography; Pediatric.
Figures
Similar articles
-
Desensitization to pegaspargase in children with acute lymphoblastic leukemia and lymphoblastic lymphoma.Pediatr Blood Cancer. 2020 Jan;67(1):e28021. doi: 10.1002/pbc.28021. Epub 2019 Oct 1. Pediatr Blood Cancer. 2020. PMID: 31571395
-
Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: A report from the Children's Oncology Group.Pediatr Blood Cancer. 2018 Mar;65(3):10.1002/pbc.26873. doi: 10.1002/pbc.26873. Epub 2017 Nov 1. Pediatr Blood Cancer. 2018. PMID: 29090524 Free PMC article. Clinical Trial.
-
PEGylated E. coli asparaginase desensitization: an effective and feasible option for pediatric patients with acute lymphoblastic leukemia who have developed hypersensitivity to pegaspargase in the absence of asparaginase Erwinia chrysanthemi availability.Pediatr Hematol Oncol. 2019 Aug;36(5):277-286. doi: 10.1080/08880018.2019.1634778. Epub 2019 Jul 12. Pediatr Hematol Oncol. 2019. PMID: 31296092 Clinical Trial.
-
Pegaspargase-induced hypertriglyceridemia in a patient with acute lymphoblastic leukemia.J Oncol Pharm Pract. 2020 Jan;26(1):193-199. doi: 10.1177/1078155219833438. Epub 2019 Mar 1. J Oncol Pharm Pract. 2020. PMID: 30823860 Review.
-
Safety, efficacy, and clinical utility of asparaginase in the treatment of adult patients with acute lymphoblastic leukemia.Onco Targets Ther. 2017 Mar 6;10:1413-1422. doi: 10.2147/OTT.S106810. eCollection 2017. Onco Targets Ther. 2017. PMID: 28331334 Free PMC article. Review.
References
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Bishop K., Kosary C.L. National Cancer Institute; Bethesda, MD: 1975-2014. SEER cancer statistics review.https://seer.cancer.gov/csr/1975_2014/ editors. based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
-
- Board PS and PCE Gastrointestinal Complications (PDQ®)–Health Professional Version. 2018. http://www.cancer.gov/about-cancer/treatment/side-effects/constipation/G... [accessed 03.18]
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

