Restoration of Glucose-Stimulated Cdc42-Pak1 Activation and Insulin Secretion by a Selective Epac Activator in Type 2 Diabetic Human Islets
- PMID: 29986926
- PMCID: PMC6152341
- DOI: 10.2337/db17-1174
Restoration of Glucose-Stimulated Cdc42-Pak1 Activation and Insulin Secretion by a Selective Epac Activator in Type 2 Diabetic Human Islets
Abstract
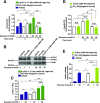
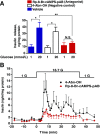
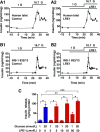
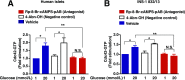
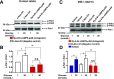
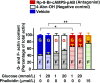
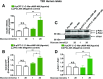
Glucose metabolism stimulates cell division control protein 42 homolog (Cdc42)-p21-activated kinase (Pak1) activity and initiates filamentous actin (F-actin) cytoskeleton remodeling in pancreatic β-cells so that cytoplasmic secretory granules can translocate to the plasma membrane where insulin exocytosis occurs. Since glucose metabolism also generates cAMP in β-cells, the cross talk of cAMP signaling with Cdc42-Pak1 activation might be of fundamental importance to glucose-stimulated insulin secretion (GSIS). Previously, the type-2 isoform of cAMP-regulated guanine nucleotide exchange factor 2 (Epac2) was established to mediate a potentiation of GSIS by cAMP-elevating agents. Here we report that nondiabetic human islets and INS-1 832/13 β-cells treated with the selective Epac activator 8-pCPT-2'-O-Me-cAMP-AM exhibited Cdc42-Pak1 activation at 1 mmol/L glucose and that the magnitude of this effect was equivalent to that which was measured during stimulation with 20 mmol/L glucose in the absence of 8-pCPT-2'-O-Me-cAMP-AM. Conversely, the cAMP antagonist Rp-8-Br-cAMPS-pAB prevented glucose-stimulated Cdc42-Pak1 activation, thereby blocking GSIS while also increasing cellular F-actin content. Although islets from donors with type 2 diabetes had profound defects in glucose-stimulated Cdc42-Pak1 activation and insulin secretion, these defects were rescued by the Epac activator so that GSIS was restored. Collectively, these findings indicate an unexpected role for cAMP as a permissive or direct metabolic coupling factor in support of GSIS that is Epac2 and Cdc42-Pak1 regulated.
© 2018 by the American Diabetes Association.
Figures

Similar articles
-
PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM in human islets of Langerhans.Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E622-33. doi: 10.1152/ajpendo.00630.2009. Epub 2009 Dec 15. Am J Physiol Endocrinol Metab. 2010. PMID: 20009023 Free PMC article.
-
Rp-cAMPS Prodrugs Reveal the cAMP Dependence of First-Phase Glucose-Stimulated Insulin Secretion.Mol Endocrinol. 2015 Jul;29(7):988-1005. doi: 10.1210/me.2014-1330. Epub 2015 Jun 10. Mol Endocrinol. 2015. PMID: 26061564 Free PMC article.
-
Phospholipase C-ε links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans.Islets. 2011 May-Jun;3(3):121-8. doi: 10.4161/isl.3.3.15507. Epub 2011 May 1. Islets. 2011. PMID: 21478675 Free PMC article.
-
Emerging Roles of Small GTPases in Islet β-Cell Function.Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review.
-
Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1.Vitam Horm. 2010;84:279-302. doi: 10.1016/B978-0-12-381517-0.00010-2. Vitam Horm. 2010. PMID: 21094904 Free PMC article. Review.
Cited by
-
Epac: A Promising Therapeutic Target for Vascular Diseases: A Review.Front Pharmacol. 2022 Jul 14;13:929152. doi: 10.3389/fphar.2022.929152. eCollection 2022. Front Pharmacol. 2022. PMID: 35910387 Free PMC article. Review.
-
Cyclic AMP-dependent protein kinase A and EPAC mediate VIP and secretin stimulation of PAK4 and activation of Na+,K+-ATPase in pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2019 Feb 1;316(2):G263-G277. doi: 10.1152/ajpgi.00275.2018. Epub 2018 Dec 6. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30520694 Free PMC article.
-
GWAS in people of Middle Eastern descent reveals a locus protective of kidney function-a cross-sectional study.BMC Med. 2022 Mar 1;20(1):76. doi: 10.1186/s12916-022-02267-7. BMC Med. 2022. PMID: 35227251 Free PMC article.
-
Metabolic regulation through the endosomal system.Traffic. 2019 Aug;20(8):552-570. doi: 10.1111/tra.12670. Epub 2019 Jun 24. Traffic. 2019. PMID: 31177593 Free PMC article. Review.
-
A glucose-dependent spatial patterning of exocytosis in human β-cells is disrupted in type 2 diabetes.JCI Insight. 2019 May 14;5(12):e127896. doi: 10.1172/jci.insight.127896. JCI Insight. 2019. PMID: 31085831 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

