Mechanism of cytokinesis failure in ovarian cystadenomas with defective BRCA1 and P53 pathways
- PMID: 29978915
- PMCID: PMC6235723
- DOI: 10.1002/ijc.31659
Mechanism of cytokinesis failure in ovarian cystadenomas with defective BRCA1 and P53 pathways
Abstract
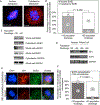
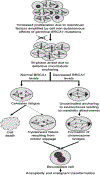
We previously described an in vitro model in which serous ovarian cystadenomas were transfected with SV40 large T antigen, resulting in loss of RB and P53 functions and thus mimicking genetic defects present in early high-grade serous extra-uterine Müllerian (traditionally called high-grade serous ovarian) carcinomas including those associated with the BRCA1 mutation carrier state. We showed that replicative aging in this cell culture model leads to a mitotic arrest at the spindle assembly checkpoint. Here we show that this arrest is due to a reduction in microtubule anchoring that coincides with decreased expression of the BUB1 kinase and of the phosphorylated form of its substrate, BUB3. The ensuing prolonged mitotic arrest leads to cohesion fatigue resulting in cell death or, in cells that recover from this arrest, in cytokinesis failure and polyploidy. Down-regulation of BRCA1 to levels similar to those present in BRCA1 mutation carriers leads to increased and uncontrolled microtubule anchoring to the kinetochore resulting in overcoming the spindle assembly checkpoint. Progression to anaphase under those conditions is associated with formation of chromatin bridges between chromosomal plates due to abnormal attachments to the kinetochore, significantly increasing the risk of cytokinesis failure. The dependence of this scenario on accelerated replicative aging can, at least in part, account for the site specificity of the cancers associated with the BRCA1 mutation carrier state, as epithelia of the mammary gland and of the reproductive tract are targets of cell-nonautonomous consequences of this carrier state on cellular proliferation associated with menstrual cycle progressions.
Keywords: BRCA1; P53; aneuploidy; cytokinesis failure; ovarian carcinoma.
© 2018 UICC.
Figures



Similar articles
-
Role of BRCA1 in controlling mitotic arrest in ovarian cystadenoma cells.Int J Cancer. 2012 Jun 1;130(11):2495-504. doi: 10.1002/ijc.26309. Epub 2011 Aug 29. Int J Cancer. 2012. PMID: 21792894 Free PMC article.
-
Pathogenesis of serous, extra-uterine Müllerian epithelial cancer and therapeutic implications.Transl Cancer Res. 2015 Feb;4(1):3-13. doi: 10.3978/j.issn.2218-676X.2015.01.06. Transl Cancer Res. 2015. PMID: 26568913 Free PMC article.
-
Analysis of the role of GSK3 in the mitotic checkpoint.Sci Rep. 2018 Sep 24;8(1):14259. doi: 10.1038/s41598-018-32435-w. Sci Rep. 2018. PMID: 30250048 Free PMC article.
-
DNA damage associated with mitosis and cytokinesis failure.Oncogene. 2013 Sep 26;32(39):4593-601. doi: 10.1038/onc.2012.615. Epub 2013 Jan 14. Oncogene. 2013. PMID: 23318447 Free PMC article. Review.
-
[Oncopathological aspects of BRCA1 and BRCA2 genes inactivation in tumors of ovary, fallopian tube and pelvic peritoneum].Cesk Patol. 2016 Fall;52(4):199-204. Cesk Patol. 2016. PMID: 27869446 Review. Czech.
Cited by
-
Toward More Comprehensive Homologous Recombination Deficiency Assays in Ovarian Cancer, Part 1: Technical Considerations.Cancers (Basel). 2022 Feb 23;14(5):1132. doi: 10.3390/cancers14051132. Cancers (Basel). 2022. PMID: 35267439 Free PMC article. Review.
-
Mechanisms of High-Grade Serous Carcinogenesis in the Fallopian Tube and Ovary: Current Hypotheses, Etiologic Factors, and Molecular Alterations.Int J Mol Sci. 2021 Apr 23;22(9):4409. doi: 10.3390/ijms22094409. Int J Mol Sci. 2021. PMID: 33922503 Free PMC article. Review.
-
Non-Surgical Cancer Risk Reduction in BRCA1 Mutation Carriers: Disabling the Remote Control.Cancers (Basel). 2020 Feb 27;12(3):547. doi: 10.3390/cancers12030547. Cancers (Basel). 2020. PMID: 32120796 Free PMC article.
References
-
- Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint Curr Biol, vol. 22, 2012:R966–R80. - PubMed
-
- Chao WC, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex Nature, vol. 484, 2012:208–13. - PubMed
-
- Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer Curr Opin Genet Dev, vol. 17, 2007:157–62. - PubMed
-
- Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol, vol. 18, 2006:658–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

