Atom-economical group-transfer reactions with hypervalent iodine compounds
- PMID: 29977394
- PMCID: PMC6009129
- DOI: 10.3762/bjoc.14.108
Atom-economical group-transfer reactions with hypervalent iodine compounds
Abstract
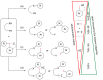
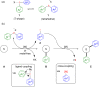
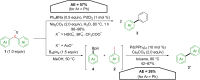
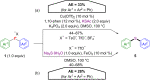
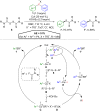
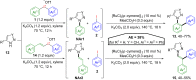
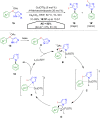
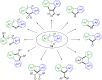
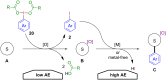
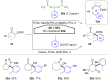
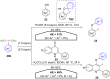
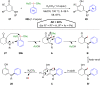
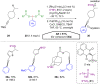
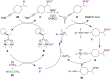
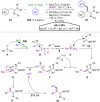
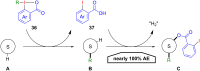
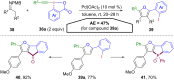
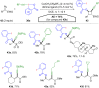
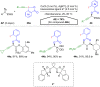
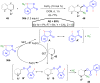
Hypervalent iodine compounds, in particular aryl-λ3-iodanes, have been used extensively as electrophilic group-transfer reagents. Even though these compounds are superior substrates in terms of reactivity and stability, their utilization is accompanied by stoichiometric amounts of an aryl iodide as waste. This highly nonpolar side product can be tedious to separate from the desired target molecules and significantly reduces the overall atom efficiency of these transformations. In this short review, we want to give a brief summary of recently developed methods, in which this arising former waste is used as an additional reagent in cascade transformations to generate multiple substituted products in one step and with high atom efficiency.
Keywords: atom economy; benziodoxolones; homogeneous catalysis; hypervalent iodine; iodonium salts.
Figures




Similar articles
-
Iodine(III) Reagents in Radical Chemistry.Acc Chem Res. 2017 Jul 18;50(7):1712-1724. doi: 10.1021/acs.accounts.7b00148. Epub 2017 Jun 21. Acc Chem Res. 2017. PMID: 28636313 Free PMC article.
-
Hypervalent iodine-guided electrophilic substitution: para-selective substitution across aryl iodonium compounds with benzyl groups.Beilstein J Org Chem. 2018 May 14;14:1039-1045. doi: 10.3762/bjoc.14.91. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 29977377 Free PMC article.
-
Hypervalent organoiodine compounds: from reagents to valuable building blocks in synthesis.Beilstein J Org Chem. 2018 Jun 21;14:1508-1528. doi: 10.3762/bjoc.14.128. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30013678 Free PMC article. Review.
-
Cyclic Hypervalent Iodine Reagents: Enabling Tools for Bond Disconnection via Reactivity Umpolung.Acc Chem Res. 2018 Dec 18;51(12):3212-3225. doi: 10.1021/acs.accounts.8b00468. Epub 2018 Nov 28. Acc Chem Res. 2018. PMID: 30485071
-
λ3 - and λ5 -Iodanes: Substituent Effects and Pseudorotation/Hypervalent Twisting.Chemistry. 2023 Jun 19;29(34):e202203997. doi: 10.1002/chem.202203997. Epub 2023 May 8. Chemistry. 2023. PMID: 36929780 Review.
Cited by
-
Synthesis of Cyclic and Acyclic ortho-Aryloxy Diaryliodonium Salts for Chemoselective Functionalizations.Chemistry. 2022 Dec 9;28(69):e202202453. doi: 10.1002/chem.202202453. Epub 2022 Oct 19. Chemistry. 2022. PMID: 36083826 Free PMC article.
-
Synthesis and reactivity of azole-based iodazinium salts.Beilstein J Org Chem. 2023 Mar 16;19:317-324. doi: 10.3762/bjoc.19.27. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 36960303 Free PMC article.
-
Thermal stability of N-heterocycle-stabilized iodanes - a systematic investigation.Beilstein J Org Chem. 2019 Sep 27;15:2311-2318. doi: 10.3762/bjoc.15.223. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31598183 Free PMC article.
-
Recent discoveries on the structure of iodine(iii) reagents and their use in cross-nucleophile coupling.Chem Sci. 2021 Jan 7;12(3):853-864. doi: 10.1039/d0sc03266b. Chem Sci. 2021. PMID: 34163852 Free PMC article. Review.
-
Recent progress in alkynylation with hypervalent iodine reagents.Chem Commun (Camb). 2023 Feb 7;59(12):1589-1604. doi: 10.1039/d2cc06168f. Chem Commun (Camb). 2023. PMID: 36656618 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
