Optical Activation of TrkA Signaling
- PMID: 29975841
- PMCID: PMC7361323
- DOI: 10.1021/acssynbio.8b00126
Optical Activation of TrkA Signaling
Abstract
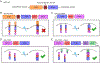
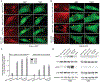
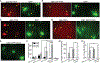
Nerve growth factor/tropomyosin receptor kinase A (NGF/TrkA) signaling plays a key role in neuronal development, function, survival, and growth. The pathway is implicated in neurodegenerative disorders including Alzheimer's disease, chronic pain, inflammation, and cancer. NGF binds the extracellular domain of TrkA, leading to the activation of the receptor's intracellular kinase domain. As TrkA signaling is highly dynamic, mechanistic studies would benefit from a tool with high spatial and temporal resolution. Here we present the design and evaluation of four strategies for light-inducible activation of TrkA in the absence of NGF. Our strategies involve the light-sensitive protein Arabidopsis cryptochrome 2 and its binding partner CIB1. We demonstrate successful recapitulation of native NGF/TrkA functions by optical induction of plasma membrane recruitment and homo-interaction of the intracellular domain of TrkA. This approach activates PI3K/AKT and Raf/ERK signaling pathways, promotes neurite growth in PC12 cells, and supports survival of dorsal root ganglion neurons in the absence of NGF. This ability to activate TrkA using light bestows high spatial and temporal resolution for investigating NGF/TrkA signaling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1.Mol Cell Biol. 2000 Nov;20(21):8069-83. doi: 10.1128/MCB.20.21.8069-8083.2000. Mol Cell Biol. 2000. PMID: 11027277 Free PMC article.
-
Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482
-
Nerve growth factor signals through TrkA, phosphatidylinositol 3-kinase, and Rac1 to inactivate RhoA during the initiation of neuronal differentiation of PC12 cells.J Biol Chem. 2002 Sep 27;277(39):35840-6. doi: 10.1074/jbc.M203617200. Epub 2002 Jul 19. J Biol Chem. 2002. PMID: 12133829
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
-
Biogenesis and function of the NGF/TrkA signaling endosome.Int Rev Cell Mol Biol. 2015;314:239-57. doi: 10.1016/bs.ircmb.2014.10.002. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619719 Free PMC article. Review.
Cited by
-
Optogenetic Approaches for the Spatiotemporal Control of Signal Transduction Pathways.Int J Mol Sci. 2021 May 18;22(10):5300. doi: 10.3390/ijms22105300. Int J Mol Sci. 2021. PMID: 34069904 Free PMC article. Review.
-
Optogenetic Control of the Canonical Wnt Signaling Pathway During Xenopus laevis Embryonic Development.J Mol Biol. 2021 Sep 3;433(18):167050. doi: 10.1016/j.jmb.2021.167050. Epub 2021 May 19. J Mol Biol. 2021. PMID: 34019868 Free PMC article.
-
Techniques for Studying Decoding of Single Cell Dynamics.Front Immunol. 2019 Apr 11;10:755. doi: 10.3389/fimmu.2019.00755. eCollection 2019. Front Immunol. 2019. PMID: 31031756 Free PMC article. Review.
-
Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions.Neural Regen Res. 2022 Jan;17(1):25-30. doi: 10.4103/1673-5374.314293. Neural Regen Res. 2022. PMID: 34100422 Free PMC article.
-
Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds.Molecules. 2020 Oct 16;25(20):4748. doi: 10.3390/molecules25204748. Molecules. 2020. PMID: 33081156 Free PMC article.
References
-
- Levi-Montalcini R, and Hamburger V (1951) Selective Growth Stimulating Effects of Mouse Sarcoma on the Sensory and Sympathetic Nervous System of the Chick Embryo. J. Exp. Zool 116 (2), 321–361. - PubMed
-
- Calissano P, Matrone C, and Amadoro G (2010) Nerve Growth Factor as a Paradigm of Neurotrophins Related to Alzheimer’s Disease. Dev. Neurobiol 70 (5), 372–383. - PubMed
-
- Cooper JD, Lindholm D, and Sofroniew MV (1994) Reduced Transport of [125I]nerve Growth Factor by Cholinergic Neurons and down-Regulated TrkA Expression in the Medial Septum of Aged Rats. Neuroscience 62 (3), 625–629. - PubMed
-
- Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, and Mufson EJ (2004) Reduction of Cortical TrkA but Not p75(NTR) Protein in Early-Stage Alzheimer’s Disease. Ann. Neurol 56 (4), 520–531. - PubMed
-
- Hirose M, Kuroda Y, and Murata E (2016) NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 16 (2), 175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

