Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study
- PMID: 29970828
- PMCID: PMC6073131
- DOI: 10.3390/ijms19071946
Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study
Abstract
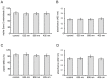
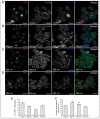
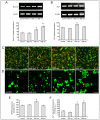
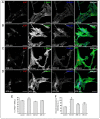
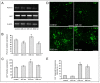
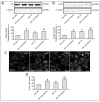
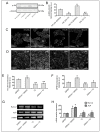
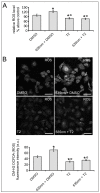
Photobiomodulation (PBM) has been used for bone regenerative purposes in different fields of medicine and dentistry, but contradictory results demand a skeptical look for its potential benefits. This in vitro study compared PBM potentiality by red (635 ± 5 nm) or near-infrared (NIR, 808 ± 10 nm) diode lasers and violet-blue (405 ± 5 nm) light-emitting diode operating in a continuous wave with a 0.4 J/cm² energy density, on human osteoblast and mesenchymal stromal cell (hMSC) viability, proliferation, adhesion and osteogenic differentiation. PBM treatments did not alter viability (PI/Syto16 and MTS assays). Confocal immunofluorescence and RT-PCR analyses indicated that red PBM (i) on both cell types increased vinculin-rich clusters, osteogenic markers expression (Runx-2, alkaline phosphatase, osteopontin) and mineralized bone-like nodule structure deposition and (ii) on hMSCs induced stress fiber formation and upregulated the expression of proliferation marker Ki67. Interestingly, osteoblast responses to red light were mediated by Akt signaling activation, which seems to positively modulate reactive oxygen species levels. Violet-blue light-irradiated cells behaved essentially as untreated ones and NIR irradiated ones displayed modifications of cytoskeleton assembly, Runx-2 expression and mineralization pattern. Although within the limitations of an in vitro experimentation, this study may suggest PBM with 635 nm laser as potential effective option for promoting/improving bone regeneration.
Keywords: Akt signaling; Runx-2; bone regeneration; diode laser; light emitting diode (LED); low level laser therapy (LLLT); mesenchymal stromal cells; osteoblasts; ostepontin; photobiomodulation (PBM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Laser and LED photobiomodulation effects in osteogenic or regular medium on rat calvaria osteoblasts obtained by newly forming bone technique.Lasers Med Sci. 2021 Apr;36(3):541-553. doi: 10.1007/s10103-020-03056-5. Epub 2020 Jun 8. Lasers Med Sci. 2021. PMID: 32514865
-
Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts.Lasers Med Sci. 2017 Apr;32(3):591-599. doi: 10.1007/s10103-017-2153-6. Epub 2017 Jan 23. Lasers Med Sci. 2017. PMID: 28116535
-
Mesenchymal stromal cell and osteoblast responses to oxidized titanium surfaces pre-treated with λ = 808 nm GaAlAs diode laser or chlorhexidine: in vitro study.Lasers Med Sci. 2017 Aug;32(6):1309-1320. doi: 10.1007/s10103-017-2243-5. Epub 2017 May 27. Lasers Med Sci. 2017. PMID: 28551763
-
Effect of Photobiomodulation on Mesenchymal Stem Cells.Photomed Laser Surg. 2016 Nov;34(11):533-542. doi: 10.1089/pho.2015.4029. Epub 2016 Apr 12. Photomed Laser Surg. 2016. PMID: 27070113 Review.
-
Presenting a Method to Improve Bone Quality Through Stimulation of Osteoporotic Mesenchymal Stem Cells by Low-Level Laser Therapy.Photomed Laser Surg. 2017 Nov;35(11):622-628. doi: 10.1089/pho.2016.4245. Epub 2017 Jun 15. Photomed Laser Surg. 2017. PMID: 28621568 Review.
Cited by
-
Optimization of a Photobiomodulation Protocol to Improve the Cell Viability, Proliferation and Protein Expression in Osteoblasts and Periodontal Ligament Fibroblasts for Accelerated Orthodontic Treatment.Biomedicines. 2024 Jan 14;12(1):180. doi: 10.3390/biomedicines12010180. Biomedicines. 2024. PMID: 38255285 Free PMC article.
-
In Vitro Cytological Responses against Laser Photobiomodulation for Periodontal Regeneration.Int J Mol Sci. 2020 Nov 26;21(23):9002. doi: 10.3390/ijms21239002. Int J Mol Sci. 2020. PMID: 33256246 Free PMC article. Review.
-
The implication of blue light-emitting diode on mesenchymal stem cells: a systematic review.Lasers Med Sci. 2023 Nov 20;38(1):267. doi: 10.1007/s10103-023-03908-w. Lasers Med Sci. 2023. PMID: 37981584
-
Selection of a suitable photosynthetically active microalgae strain for the co-cultivation with mammalian cells.Front Bioeng Biotechnol. 2022 Sep 19;10:994134. doi: 10.3389/fbioe.2022.994134. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36199362 Free PMC article.
-
The Effects and Mechanisms of PBM Therapy in Accelerating Orthodontic Tooth Movement.Biomolecules. 2023 Jul 17;13(7):1140. doi: 10.3390/biom13071140. Biomolecules. 2023. PMID: 37509176 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

