CD8+ T cells specific for conserved, cross-reactive Gag epitopes with strong ability to suppress HIV-1 replication
- PMID: 29970102
- PMCID: PMC6029025
- DOI: 10.1186/s12977-018-0429-y
CD8+ T cells specific for conserved, cross-reactive Gag epitopes with strong ability to suppress HIV-1 replication
Abstract
Background: Development of AIDS vaccines for effective prevention of circulating HIV-1 is required, but no trial has demonstrated definitive effects on the prevention. Several recent T-cell vaccine trials showed no protection against HIV-1 acquisition although the vaccines induced HIV-1-specific T-cell responses, suggesting that the vaccine-induced T cells have insufficient capacities to suppress HIV-1 replication and/or cross-recognize circulating HIV-1. Therefore, it is necessary to develop T-cell vaccines that elicit T cells recognizing shared protective epitopes with strong ability to suppress HIV-1. We recently designed T-cell mosaic vaccine immunogens tHIVconsvX composed of 6 conserved Gag and Pol regions and demonstrated that the T-cell responses to peptides derived from the vaccine immunogens were significantly associated with lower plasma viral load (pVL) and higher CD4+ T-cell count (CD4 count) in HIV-1-infected, treatment-naive Japanese individuals. However, it remains unknown T cells of which specificities have the ability to suppress HIV-1 replication. In the present study, we sought to identify more T cells specific for protective Gag epitopes in the vaccine immunogens, and analyze their abilities to suppress HIV-1 replication and recognize epitope variants in circulating HIV-1.
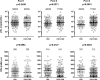
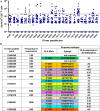
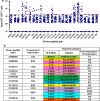
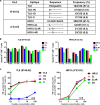
Results: We determined 17 optimal Gag epitopes and their HLA restriction, and found that T-cell responses to 9 were associated significantly with lower pVL and/or higher CD4 count. T-cells recognizing 5 of these Gag peptides remained associated with good clinical outcome in 221 HIV-1-infected individuals even when comparing responders and non-responders with the same restricting HLA alleles. Although it was known previously that T cells specific for 3 of these protective epitopes had strong abilities to suppress HIV-1 replication in vivo, here we demonstrated equivalent abilities for the 2 novel epitopes. Furthermore, T cells against all 5 Gag epitopes cross-recognized variants in majority of circulating HIV-1.
Conclusions: We demonstrated that T cells specific for 5 Gag conserved epitopes in the tHIVconsvX have ability to suppress replication of circulating HIV-1 in HIV-1-infected individuals. Therefore, the tHIVconsvX vaccines have the right specificity to contribute to prevention of HIV-1 infection and eradication of latently infected cells following HIV-1 reactivation.
Keywords: CTL; Conserved epitope; Gag; HIV-1; Vaccine.
Figures



Similar articles
-
Effective Suppression of HIV-1 Replication by Cytotoxic T Lymphocytes Specific for Pol Epitopes in Conserved Mosaic Vaccine Immunogens.J Virol. 2019 Mar 21;93(7):e02142-18. doi: 10.1128/JVI.02142-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30674626 Free PMC article.
-
Clinical Control of HIV-1 by Cytotoxic T Cells Specific for Multiple Conserved Epitopes.J Virol. 2015 May;89(10):5330-9. doi: 10.1128/JVI.00020-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25741000 Free PMC article.
-
Broad Recognition of Circulating HIV-1 by HIV-1-Specific Cytotoxic T-Lymphocytes with Strong Ability to Suppress HIV-1 Replication.J Virol. 2018 Dec 10;93(1):e01480-18. doi: 10.1128/JVI.01480-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30333175 Free PMC article.
-
Identification of highly conserved and broadly cross-reactive HIV type 1 cytotoxic T lymphocyte epitopes as candidate immunogens for inclusion in Mycobacterium bovis BCG-vectored HIV vaccines.AIDS Res Hum Retroviruses. 2000 Sep 20;16(14):1433-43. doi: 10.1089/08892220050140982. AIDS Res Hum Retroviruses. 2000. PMID: 11018863 Review.
-
Unusual antigen presentation offers new insight into HIV vaccine design.Curr Opin Immunol. 2017 Jun;46:75-81. doi: 10.1016/j.coi.2017.04.009. Epub 2017 May 12. Curr Opin Immunol. 2017. PMID: 28505602 Free PMC article. Review.
Cited by
-
Tetravalent Immunogen Assembled from Conserved Regions of HIV-1 and Delivered as mRNA Demonstrates Potent Preclinical T-Cell Immunogenicity and Breadth.Vaccines (Basel). 2020 Jul 6;8(3):360. doi: 10.3390/vaccines8030360. Vaccines (Basel). 2020. PMID: 32640600 Free PMC article.
-
Identification of a Clade-Specific HLA-C*03:02 CTL Epitope GY9 Derived from the HIV-1 p17 Matrix Protein.Int J Mol Sci. 2024 Sep 6;25(17):9683. doi: 10.3390/ijms25179683. Int J Mol Sci. 2024. PMID: 39273630 Free PMC article.
-
Parallel Induction of CH505 B Cell Ontogeny-Guided Neutralizing Antibodies and tHIVconsvX Conserved Mosaic-Specific T Cells against HIV-1.Mol Ther Methods Clin Dev. 2019 Jun 29;14:148-160. doi: 10.1016/j.omtm.2019.06.003. eCollection 2019 Sep 13. Mol Ther Methods Clin Dev. 2019. PMID: 31367651 Free PMC article.
-
HIV-1 protective epitope-specific CD8+ T cells in HIV-1-exposed seronegative individuals.iScience. 2023 Sep 29;26(11):108089. doi: 10.1016/j.isci.2023.108089. eCollection 2023 Nov 17. iScience. 2023. PMID: 37867946 Free PMC article.
-
HLA-E-restricted HIV-1-specific CD8+ T cell responses in natural infection.J Clin Invest. 2021 Aug 16;131(16):e148979. doi: 10.1172/JCI148979. J Clin Invest. 2021. PMID: 34228645 Free PMC article.
References
-
- Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 15fk0410019h0001/Japan Agency for Medical Research and Development/International
- 17fk0410302h0003/Japan Agency for Medical Research and Development/International
- 17K10021/Japan Society for the Promotion of Science/International
- 26293240/Japan Society for the Promotion of Science/International
- 16fk0410202h0002/Japan Agency for Medical Research and Development/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

