Similar Albeit Not the Same: In-Depth Analysis of Proteoforms of Human Serum, Bovine Serum, and Recombinant Human Fetuin
- PMID: 29966421
- PMCID: PMC6079914
- DOI: 10.1021/acs.jproteome.8b00318
Similar Albeit Not the Same: In-Depth Analysis of Proteoforms of Human Serum, Bovine Serum, and Recombinant Human Fetuin
Abstract
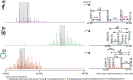
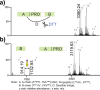
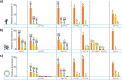
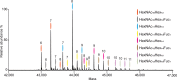
Fetuin, also known as alpha-2-Heremans Schmid glycoprotein (AHSG), belongs to some of the most abundant glycoproteins secreted into the bloodstream. In blood, fetuins exhibit functions as carriers of metals and small molecules. Bovine fetuin, which harbors 3 N-glycosylation sites and a suggested half dozen O-glycosylation sites, has been used often as a model glycoprotein to test novel analytical workflows in glycoproteomics. Here we characterize and compare fetuin in depth, using protein from three different biological sources: human serum, bovine serum, and recombinant human fetuin expressed in HEK-293 cells, with the aim to elucidate similarities and differences between these proteins and the post-translational modifications they harbor. Combining data from high-resolution native mass spectrometry and glycopeptide centric LC-MS analysis, we qualitatively and quantitatively gather information on fetuin protein maturation, N-glycosylation, O-glycosylation, and phosphorylation. We provide direct experimental evidence that both the human serum and part of the recombinant proteins are processed into two chains (A and B) connected by a single interchain disulfide bridge, whereas bovine fetuin remains a single-chain protein. Although two N-glycosylation sites, one O-glycosylation site, and a phosphorylation site are conserved from bovine to human, the stoichiometry of the modifications and the specific glycoforms they harbor are quite distinct. Comparing serum and recombinant human fetuin, we observe that the serum protein harbors a much simpler proteoform profile, indicating that the recombinant protein is not ideally engineered to mimic human serum fetuin. Comparing the proteoform profile and post-translational modifications of human and bovine serum fetuin, we observe that, although the gene structures of these two proteins are alike, they represent quite distinct proteins when their glycoproteoform profile is also taken into consideration.
Keywords: N-glycosylation; O-glycosylation; alpha-2-HS glycoprotein; fetuin; glycopeptides; glycoprotein; hybrid mass spectrometry; native mass spectrometry; proteoforms; serum proteins.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Glycoproteogenomics: A Frequent Gene Polymorphism Affects the Glycosylation Pattern of the Human Serum Fetuin/α-2-HS-Glycoprotein.Mol Cell Proteomics. 2019 Aug;18(8):1479-1490. doi: 10.1074/mcp.RA119.001411. Epub 2019 May 16. Mol Cell Proteomics. 2019. PMID: 31097672 Free PMC article.
-
Site-specific analysis of the O-glycosylation of bovine fetuin by electron-transfer dissociation mass spectrometry.J Proteomics. 2014 Aug 28;108:258-68. doi: 10.1016/j.jprot.2014.05.022. Epub 2014 Jun 4. J Proteomics. 2014. PMID: 24907489
-
Post-translational modifications glycosylation and phosphorylation of the major hepatic plasma protein fetuin-A are associated with CNS inflammation in children.PLoS One. 2022 Oct 7;17(10):e0268592. doi: 10.1371/journal.pone.0268592. eCollection 2022. PLoS One. 2022. PMID: 36206263 Free PMC article.
-
From infancy to aging: Biological and behavioral modifiers of Fetuin-A.Biochimie. 2016 May;124:141-149. doi: 10.1016/j.biochi.2015.12.016. Epub 2015 Dec 29. Biochimie. 2016. PMID: 26740309 Review.
-
Methods in enzymology: O-glycosylation of proteins.Methods Enzymol. 2005;405:139-71. doi: 10.1016/S0076-6879(05)05007-X. Methods Enzymol. 2005. PMID: 16413314 Review.
Cited by
-
[Applications of native mass spectrometry and ultraviolet photodissociation in protein structure and interaction analysis].Se Pu. 2024 Jul;42(7):681-692. doi: 10.3724/SP.J.1123.2024.01021. Se Pu. 2024. PMID: 38966976 Free PMC article. Review. Chinese.
-
QTQTN motif upstream of the furin-cleavage site plays a key role in SARS-CoV-2 infection and pathogenesis.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2205690119. doi: 10.1073/pnas.2205690119. Epub 2022 Jul 26. Proc Natl Acad Sci U S A. 2022. PMID: 35881779 Free PMC article.
-
Detection and Characterization of Phosphorylation, Glycosylation, and Fatty Acid Bound to Fetuin A in Human Blood.J Clin Med. 2021 Jan 22;10(3):411. doi: 10.3390/jcm10030411. J Clin Med. 2021. PMID: 33499061 Free PMC article.
-
The structure, biosynthesis, and biological roles of fetuin-A: A review.Front Cell Dev Biol. 2022 Jul 18;10:945287. doi: 10.3389/fcell.2022.945287. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35923855 Free PMC article. Review.
-
H3N2 influenza A virus gradually adapts to human-type receptor binding and entry specificity after the start of the 1968 pandemic.Proc Natl Acad Sci U S A. 2023 Aug;120(31):e2304992120. doi: 10.1073/pnas.2304992120. Epub 2023 Jul 19. Proc Natl Acad Sci U S A. 2023. PMID: 37467282 Free PMC article.
References
-
- Bürgi W.; Schmid K. Preparation and Properties of Zn-Alpha 2-Glycoprotein of Normal Human Plasma. J. Biol. Chem. 1961, 236 (4), 1066–1074. - PubMed
-
- Heremans J. F.Les Globulines Du Système Gamma Du Plasma Humain; Editions Arscia S.A. Paris: Brussels, 1961; pp 119–138; 10.5169/seals-307477. - DOI
-
- Pedersen K. O. Fetuin, a New Globulin Isolated from Serum. Nature 1944, 154, 575.10.1038/154575a0. - DOI
-
- Dziegielewska K. M.; Brown W. M.; Casey S. J.; Christie D. L.; Foreman R. C.; Hill R. M.; Saunders N. R. The Complete cDNA and Amino Acid Sequence of Bovine Fetuin. Its homology with alpha 2HS glycoprotein and relation to other members of the cystatin superfamily. J. Biol. Chem. 1990, 265 (6), 4354–4357. - PubMed
-
- Brown W. M.; Dziegielewska K. M.; Saunders N. R.; Christie D. L.; Nawratil P.; Müller-Esterl W. The Nucleotide and Deduced Amino Acid Structures of Sheep and Pig Fetuin: Common Structural Features of the Mammalian Fetuin Family. Eur. J. Biochem. 1992, 205 (1), 321–331. 10.1111/j.1432-1033.1992.tb16783.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

